Triphenylphosphine oxide unit bridged bipolar host material and application thereof
A technology of triphenylphosphine oxide and host materials, which is applied in the fields of compounds of group 5/15 elements of the periodic table, electrical components, and electric solid-state devices. Limited solubility and other issues, to achieve strong electron transport capabilities, improve hole transport capabilities, and enhance rigidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
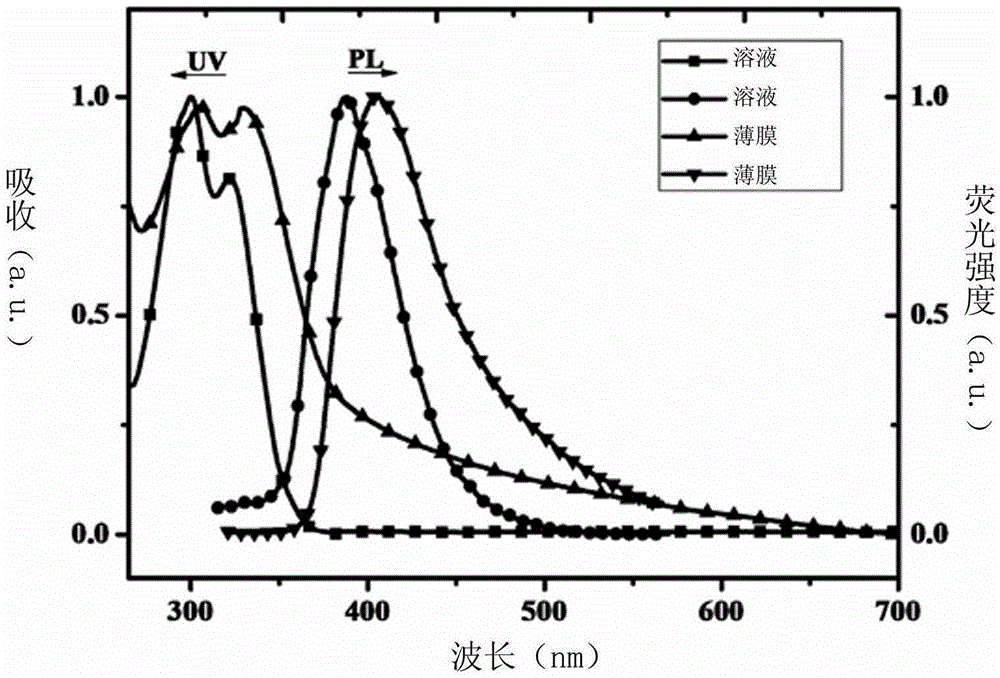
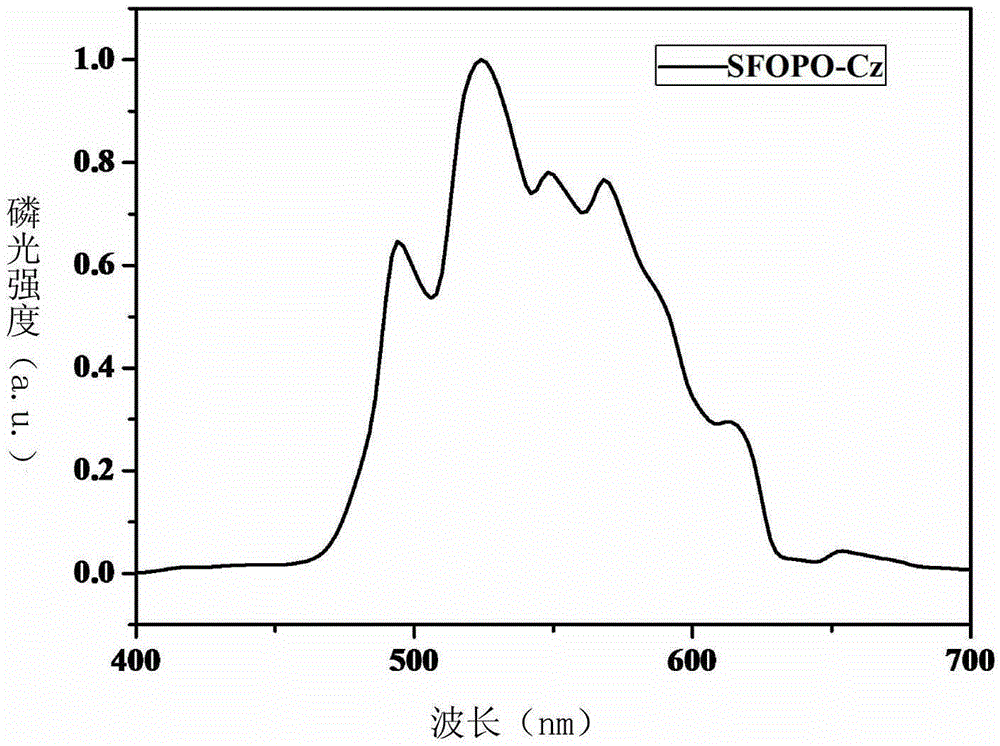
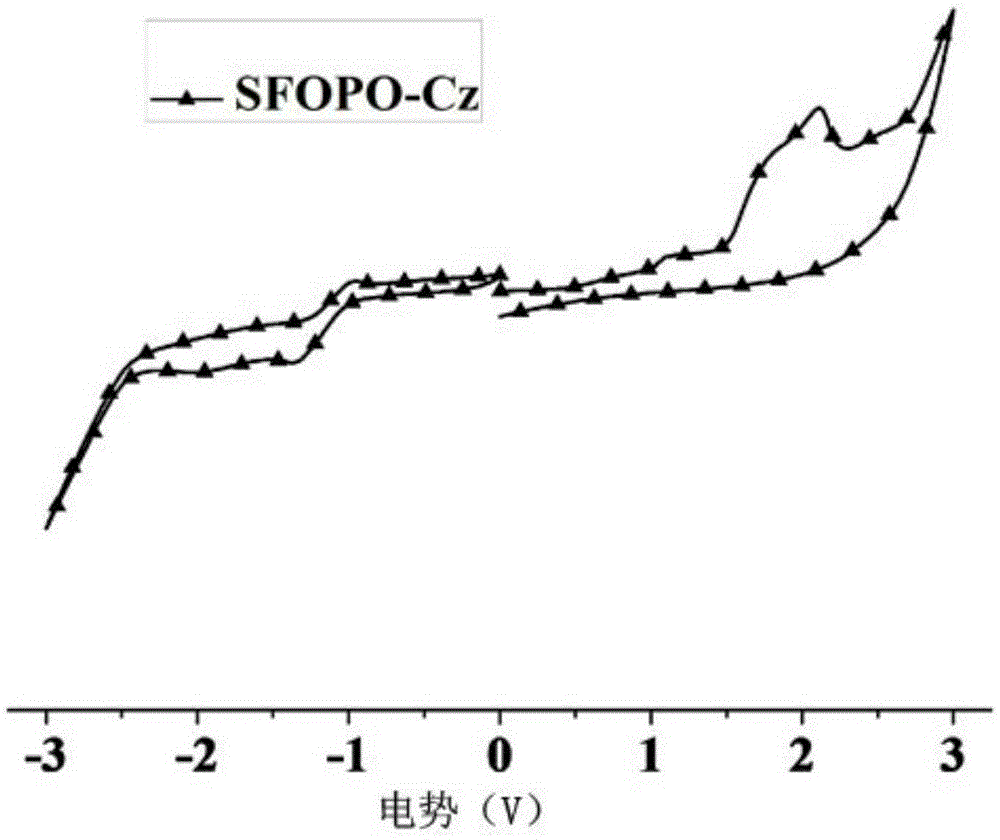
[0032] Embodiment 1: the synthesis of SFOPO-Cz
[0033] Synthesis of 2-bromospiro[fluorene-9,9'-oxanthene]
[0034] Methanesulfonic acid (2.14 mL, 24.4 mmol), 2-bromo-9-fluorenone (6 g, 24.63 mmol) and phenol (23.15 g, 246.3 mmol) were added into a two-necked flask. After pumping 3 times in N 2 In the atmosphere, react at a temperature of 150°C for 8h. The reaction substrate was quenched with water, extracted three times with dichloromethane and then washed with MgSO 4 dry. TLC analysis yielded 6.1 g (60%) of pure product. 1 H-NMR (400MHz), CDCl 3 ,δ:δ7.78(s,1H),δ7.66(s,1H),δ7.50(s,1H),δ7.38(m,1H),δ7.3-7.15(m,7H), δ6.79(m,2H), δ6.40(s,2H).
[0035] Synthesis of Bis(4-bromobenzene)(phenyl)phosphine oxide
[0036] First at N 2 Under atmosphere, 1,4-dibromobenzene (4.72 g, 20 mmol) was dissolved in 140 ml of dehydrated tetrahydrofuran solvent in a two-necked flask. At a temperature of -78°C, 1.6 mol / L n-butyllithium (11.865 mL, 19 mmol) was added dropwise. After reacti...
Embodiment 2
[0051] Example 2: Electrophosphorescent device with SFOPO-Cz as host material
[0052] We used SFOPO-Cz as the host material, and the guest material Ir(ppy) 3 ,Ir(pq) 2 (acac) doping prepared electroluminescent red and green light devices, the device structure is: ITO / MoO 3 / CBP / EML / TPBi / LiF / Al, the specific steps for preparing electroluminescent devices:
[0053] (1) The ITO electrode glass plate is ultrasonically treated with a cleaning agent, then rinsed in deionized water, then ultrasonically treated with acetone-ethanol (1:3) mixed solvent to remove oil, and baked in a clean environment until the water is completely removed. Irradiate with a UV light cleaner for 10 minutes;
[0054] (2) evaporate the CBP layer on the above-mentioned ITO glass plate as the hole injection layer, the evaporation rate is 0.1nm / s, and the evaporation thickness is 45nm;
[0055] (3) Place the above-mentioned ITO glass covered with the CBP layer in a vacuum chamber and vacuumize.
[0056] (...
Embodiment 3
[0060] Example 3: Application of organic electroluminescent materials in the preparation of electroluminescent devices
[0061] The compounds of the present invention can be used as electroluminescent materials to prepare electroluminescent devices, especially for active layers of electroluminescent devices. The so-called active layer is an organic thin film layer that can emit light or have charge injection and transport properties under a certain driving voltage. The active layer can be a hole transport layer, an organic light-emitting layer, or an electron transport layer. Electroluminescent devices have one or more active layers. The compound of the present invention can be used as an electroluminescent material, preferably mixed with other dyes as a light-emitting layer.
[0062] The basic structure of organic electroluminescence is: substrate / anode / hole injection layer / hole transport layer / hole blocking layer / organic light-emitting layer / electron transport layer / electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com