Production method of recombinant human fibroblast growth factor-17
A technology of human fibroblasts and growth factors, which is applied in the field of genetic engineering, can solve the problems of low production concentration and purity of FGF-17 protein, and achieve the effects of optimizing culture temperature and increasing purity and concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Construction of FGF-17 Protein High Expression Engineering Bacteria
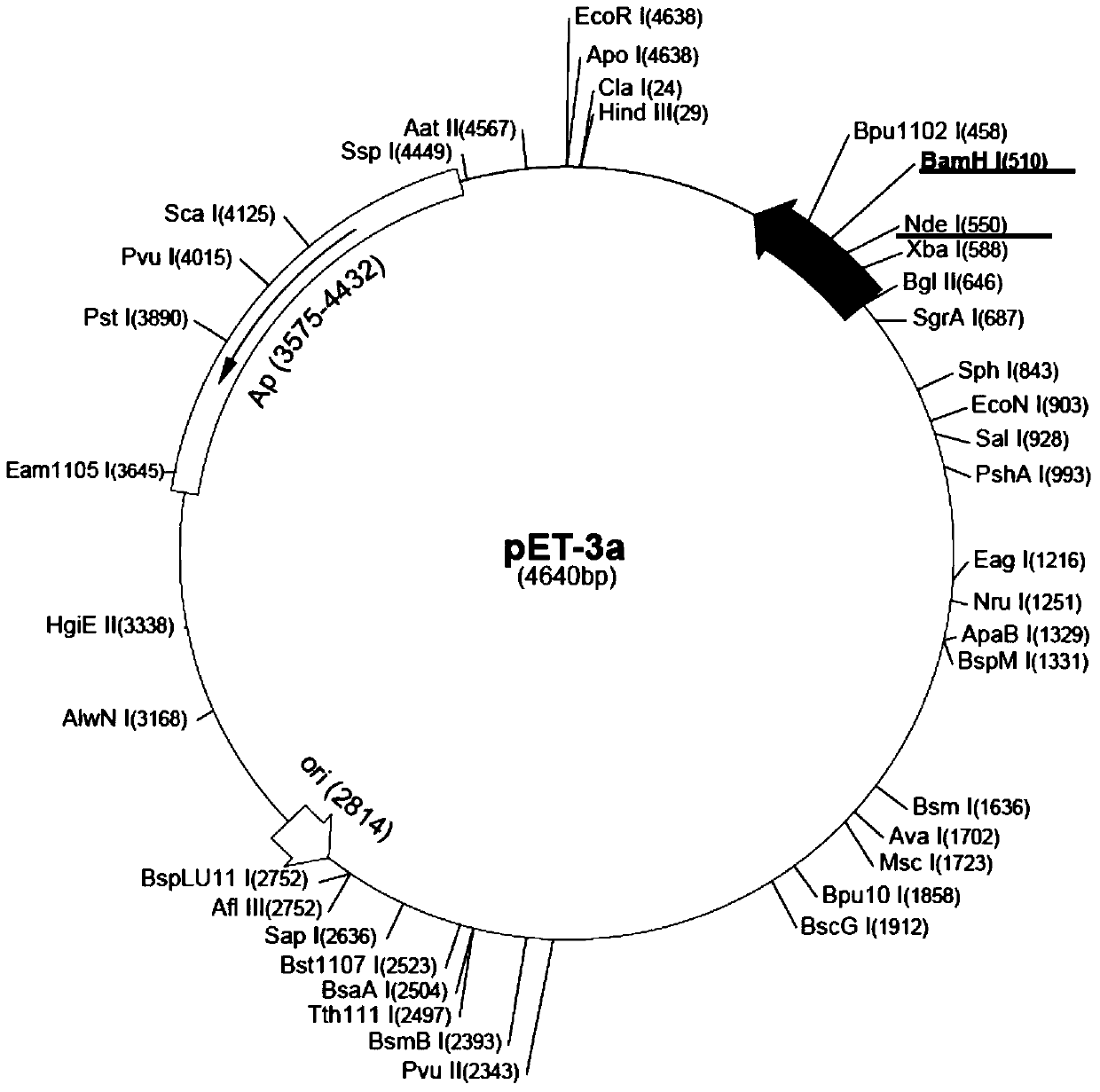
[0072] According to the human FGF17 natural sequence (accession number: AY358869.1) and amino acid sequence published in GenBank, optimize according to the codon preference of Escherichia coli, under the condition of not changing the amino acid sequence, design the coding sequence primer of rhFGF-17, and Introduce specific enzyme cutting sites NdeI and BamHI. The nucleotide sequence of rhFGF-17 was obtained by PCR amplification, and the resulting product was ligated with the T vector (pEASY T1 simple vector) at 16°C, and the ligated product was digested with NdeI and BamHI at 37°C for 4 hours, and rhFGF-17 was recovered. 17 gene fragments, then treated with NdeI and BamHI double enzyme digestion and recovered expression vector pET3a gene fragments were ligated overnight at 16°C with Solution I ligase to construct recombinant expression vectors (see figure 1 , figure 2 ). The ligation pro...
Embodiment 2
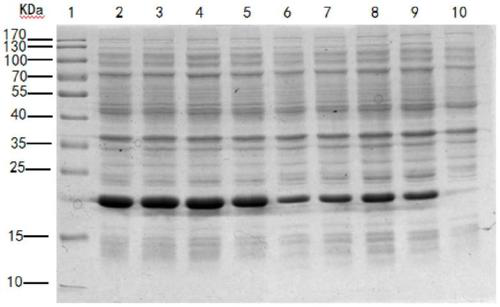
[0073] Example 2 Establishment of a higher density culture method for FGF-17 protein
[0074] In the present invention, tryptone, yeast powder, etc. are used as nitrogen sources, glucose, etc. are used as carbon sources, and phosphate is used as a buffer system, supplemented by sodium chloride as a base medium; and by controlling conditions (pH, temperature, dissolving Oxygen, rotation number, etc.) significantly improved the cell yield and expression level. Specifically: Inoculate the FGF-17 engineering strain into LB medium at a ratio of 1:50-100 to obtain the first-generation bacteria, cultivate for 8h-12h, and wait for A 600 When it reaches about 1.2-2.0, it is inoculated into LB medium containing phosphate buffered saline at a ratio of 1:100-200 for cultivation, and cultured for 2h-4h. 600 When the temperature reaches 0.6-0.8, the temperature is 16°C and 37°C, the final concentration of the inducer (IPTG) is 0.4mM and 1mM, the time is 16-30h and 4-6h, and the rotation sp...
Embodiment 3
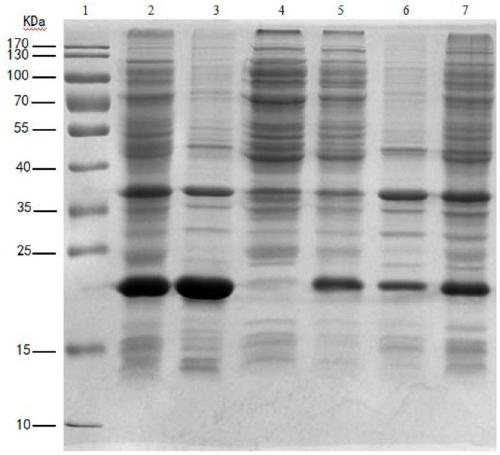
[0077] Example 3 Establishment of FGF-17 Protein Purification Method
[0078] 1. Treatment of inclusion bodies
[0079] (1) Bacterial fragmentation
[0080] After freezing and thawing the cells repeatedly, they were fully suspended in cell lysis buffer (20mM Tris-HCl, 1mM EDTA-2Na, 0.2M NaCl, 1% Triton X-100, 0.2% Sodium oxycholate, pH7.5), perform low-temperature ultrasonic crushing, power 300w, vibration frequency output 40%, ultrasonic 5s off 5s, ultrasonic 10min, microscopic examination of E. , 4°C, 25min, discard the supernatant, and collect the precipitate.
[0081] (2) Inclusion body cleaning
[0082] Take the precipitate after centrifugation, add 20 times the amount of washing buffer (20mM Tris-HCl, 1mM EDTA-2Na, 0.2M NaCl, 1% Triton X-100, pH 7.5) to fully stir and wash, after 30min, centrifuge at 20000rpm, 4°C, 25min, discard the supernatant, and collect the precipitate. Then take the precipitate, add 20 times the amount of washing buffer (20mM Tris-HCl, 1mM EDTA-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com