Method for enhancing electrochemical activity of polyaniline in neutral medium
A neutral medium and activity-enhancing technology, applied in the field of electrochemistry, can solve the problems of unsatisfactory polyaniline and poor cycle stability, and achieve the effects of improving electrochemical activity, increasing specific surface area, and increasing surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Firstly, TiO was prepared by constant-voltage secondary anodic oxidation method. 2 Electrode: Anodic oxidation is carried out in a two-electrode system with titanium foil as the working electrode and carbon rod as the counter electrode. The electrolyte is 0.5wt% NH 4 F and 2vol%H 2 O solution in ethylene glycol. First, the titanium foil was ultrasonically cleaned with acetone, ethanol and water for 10 min in sequence. The first anodic oxidation was carried out at a constant voltage of 60V for 2h, and then the oxide film was removed by ultrasonication for 30min, and the pitted titanium foil was cleaned with acetone, ethanol and water in sequence. Then carry out the second anodic oxidation, the oxidation conditions are the same as the first oxidation, the anodic oxidation time is 30min, and the TiO is taken out after the oxidation is completed. 2 The electrodes were rinsed with deionized water and dried. The prepared amorphous TiO 2 The electrode is an ordered nanotu...
Embodiment 2
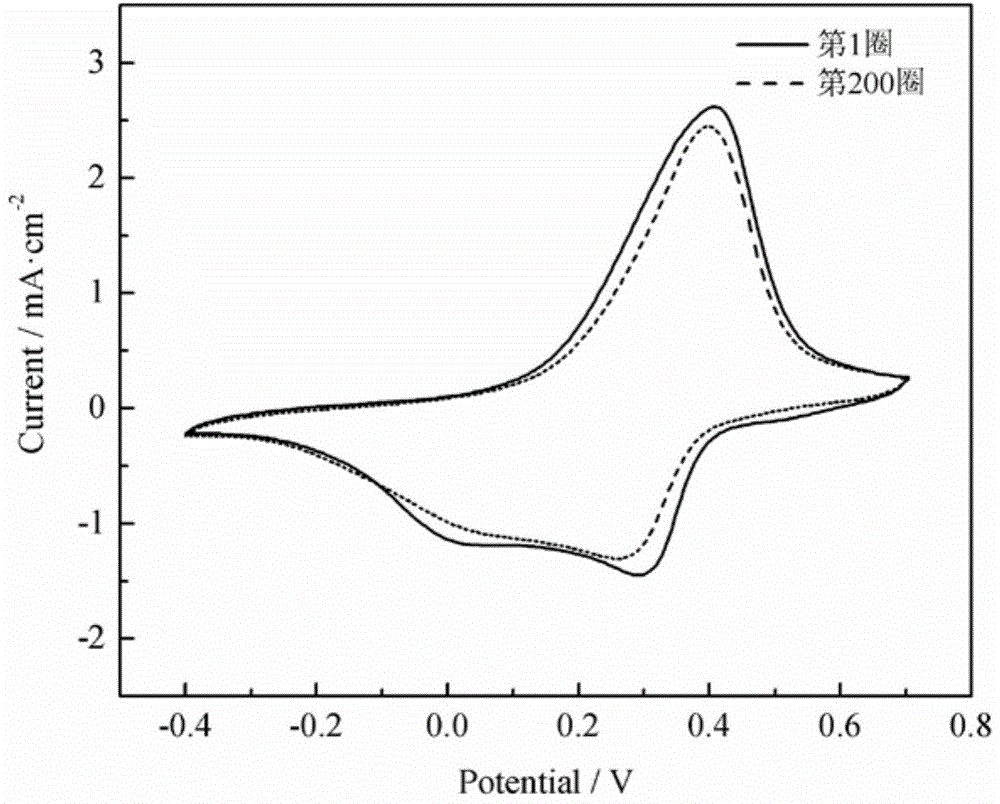
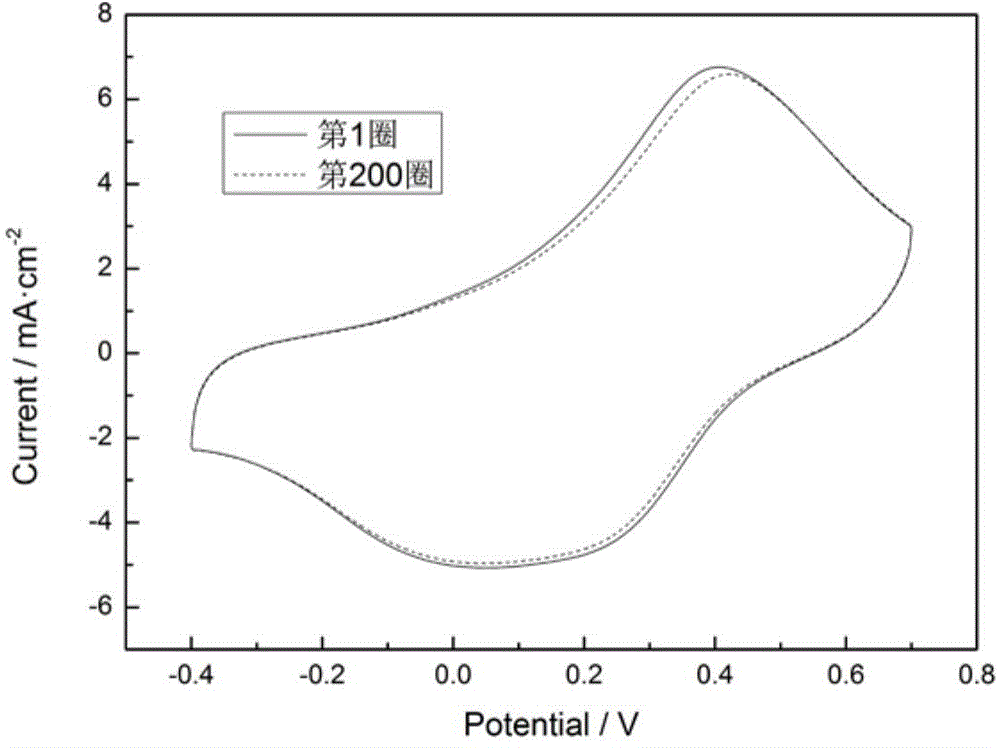
[0029] Except changing the secondary anodic oxidation time of titanium foil to 10 min; the electrochemical deposition time of tungsten oxide to 150 s; and the number of scanning circles for polyaniline polymerization to be 5 circles, all other materials and process conditions are the same as in Example 1. The test method of the cyclic voltammetry performance of the polyaniline film is also the same as in Example 1. The results show that the strong peaks at around 0.4V and 0.2V on the cyclic voltammetry curve correspond to the redox process of polyaniline, and the peak current is significantly higher than that of Comparative Example 1, indicating that the polyaniline film has a significantly enhanced electrochemical activity. After 200 cycles of continuous scanning, the electrode still has stable electrochemical activity, and the peak current and peak position remain basically unchanged compared with the first cycle.
Embodiment 3
[0031] Except changing the titanium foil anodizing voltage to 15V; the secondary anodizing time to 10min; the electrochemical deposition time of tungsten oxide to 400s; the number of scan cycles for polyaniline polymerization to be 15, all other materials and process conditions are the same as in Example 1 . The test method of the cyclic voltammetry performance of the polyaniline film is also the same as in Example 1. The results show that the strong peaks at around 0.4V and 0.2V on the cyclic voltammetry curve correspond to the redox process of polyaniline, and the peak current is significantly higher than that of Comparative Example 1, indicating that the polyaniline film has a significantly enhanced electrochemical activity. After 200 cycles of continuous scanning, the electrode still has stable electrochemical activity, and the peak current and peak position remain basically unchanged compared with the first cycle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com