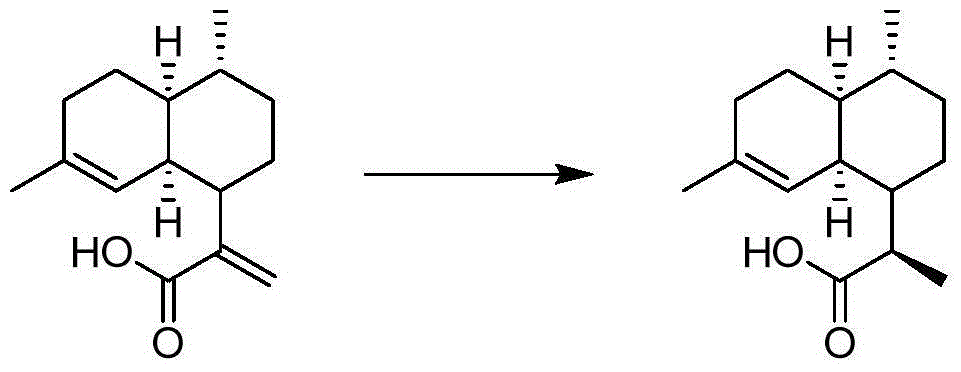
Asymmetric synthesis method of dihydroartemisinic acid from arteannuinic acid
A technology of dihydroartemisinic acid and synthesis method, which is applied in the direction of ozone oxidation to prepare carboxylic acid, organic chemistry, etc., can solve the problems of destroying the environment and ecological balance, resource depletion, time-consuming and labor-intensive, etc., and achieve good industrial application prospects and cost advantages Obvious, simple synthetic process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
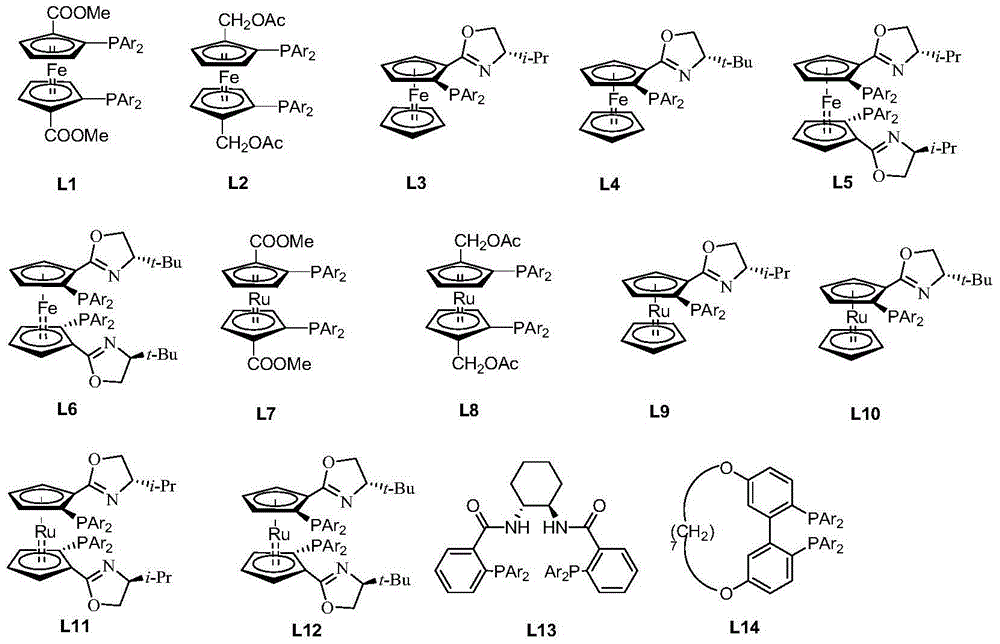
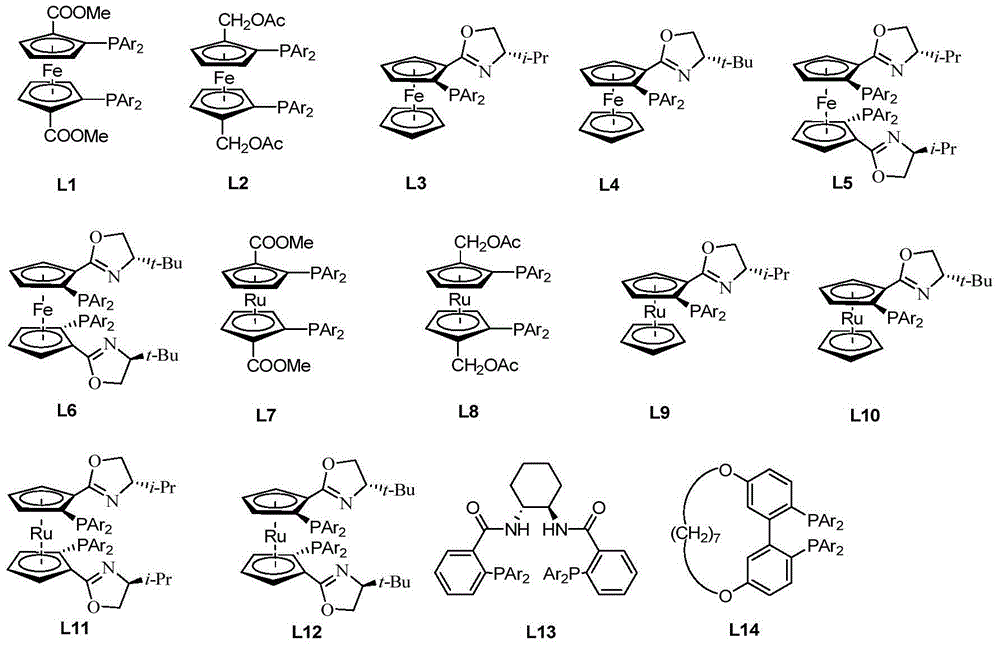
[0046] 1.1 Synthesis of chiral catalysts
[0047] Tris(triphenylphosphine)ruthenium dichloride (3.8 mg, 4 μmol) and chiral ligand L11b (1.76 mg, 2 μmol) were dissolved in methanol (3 mL), heated and stirred at 30° C. for 1 hour. Cool to room temperature, remove the solvent under reduced pressure, and separate by column chromatography (using silica gel column, eluent: ethyl acetate / petroleum ether=1 / 5) to obtain 3.21 mg of a dark green solid with a yield of 92%.
[0048] 1.2 Asymmetric synthesis of dihydroartemisinic acid
[0049]
[0050] Under nitrogen atmosphere, artemisinic acid (0.4mmol), methanol (2.6mL), potassium hydroxide in methanol (0.4mL, 0.05M) and catalyst 0.69mg (TON=1000). The reaction system was placed in an autoclave at 25 °C and H 2 (20atm) and stirred for 6 hours. The solvent was removed under reduced pressure and separated by column chromatography (using silica gel column, eluent: ethyl acetate / petroleum ether = 1 / 1) to obtain 0.091 g of pure product,...
Embodiment 2
[0053] 2.1 Synthesis of chiral catalysts
[0054] Tris(triphenylphosphine)ruthenium dichloride (3.8 mg, 4 μmmol) and chiral ligand L11a (2.3 mg, 2.8 μmmol) were dissolved in ethanol (3 mL), heated and stirred at 70° C. for 3 hours. Cool to room temperature, remove the solvent under reduced pressure, and separate by column chromatography (using a silica gel column, eluent: ethyl acetate / petroleum ether=1 / 5) to obtain 3.31 mg of a dark green solid with a yield of 94.8%.
[0055] 2.2 Asymmetric synthesis of dihydroartemisinic acid
[0056] Under nitrogen atmosphere, artemisinic acid (0.4mmol), ethanol (2.6mL) and ethanol solution of potassium hydroxide (0.4mL, 0.07M) and catalyst 0.63mg (TON=1000) were added. The reaction system was placed in an autoclave at 25 °C and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressure and separated by column chromatography (using silica gel column, eluent: ethyl acetate / petroleum ether = 1 / 1) to obtain 0.092 g...
Embodiment 3
[0059] 3.1 Synthesis of chiral catalysts
[0060] Tris(triphenylphosphine)ruthenium dichloride (3.8 mg, 4 μmmol) and chiral ligand L2a (1.8 mg, 2.6 μmmol) were dissolved in toluene (3 mL), heated and stirred at 100° C. for 4 hours. Cool to room temperature, remove the solvent under reduced pressure, and separate by column chromatography (using a silica gel column, eluent: ethyl acetate / petroleum ether=1 / 5) to obtain 3.56 mg of a dark green solid with a yield of 96.1%.
[0061] 3.2 Asymmetric synthesis of dihydroartemisinic acid
[0062] Under nitrogen atmosphere, artemisinic acid (0.4mmol), toluene (2.6mL), potassium hydroxide methanol solution (0.4mL, 0.1M) and the above catalyst 0.32mg (TON=2000) were added. The reaction system was placed in an autoclave at 25 °C and H 2 (20atm) and stirred for 12 hours. The solvent was removed under reduced pressure and separated by column chromatography (using silica gel column, eluent: ethyl acetate / petroleum ether = 1 / 1) to obtain 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com