Hexahydrobenzonaphthyridine-type optically active compound and pharmaceutical use thereof
A technology for hexahydrobenzene and compound, which is applied in the field of hexahydrobenzonaphthyridine optically active compounds and their pharmaceutical uses, and can solve the problems that biological activity and therapeutic effect have not been studied and revealed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Racemic 5-amino-4-(2-chlorophenyl)-2,7,7-trimethyl-1,4,6,7,8,9-hexahydrobenzo[b][ Preparation of ethyl 1,8]naphthyridine-3-carboxylate (compound T)
[0031]
[0032] Synthesis of Step 1 Raw Material A
[0033] Mix and dissolve malononitrile (330g, 5mol) and absolute ethanol (460.7g, 10mol) in 1.5L ethyl acetate, stir evenly at room temperature, add acetyl chloride (392.5g, 5mol) dropwise under ice bath, dropwise React overnight below 5°C, a large amount of solids precipitate out, filter, wash, and dry to obtain A600g, yield 80.76%
[0034] Synthesis of Step 2 Compound B
[0035] Dissolve 2-chlorobenzaldehyde (421.5g, 3mol) and ethyl acetoacetate (390.4g, 3mol) in 1L of n-hexane, add acetic acid (9.01g, 0.15mol) and piperidine (12.77g, 0.15mol), After stirring and refluxing with water for 3 hours, TLC monitored the completion of the reaction, cooled and concentrated to obtain product B, 690.2 g of light yellow oil, with a yield of 91.04%.
[0036] Synth...
Embodiment 2
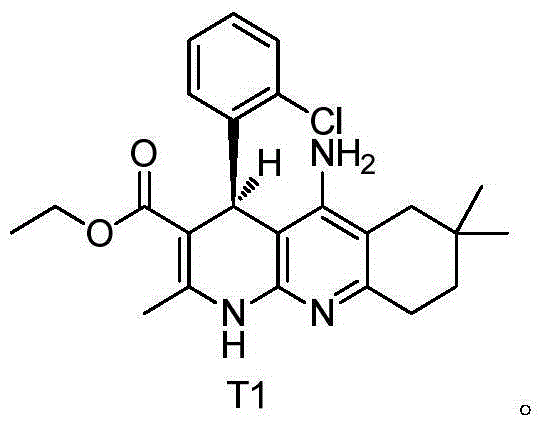
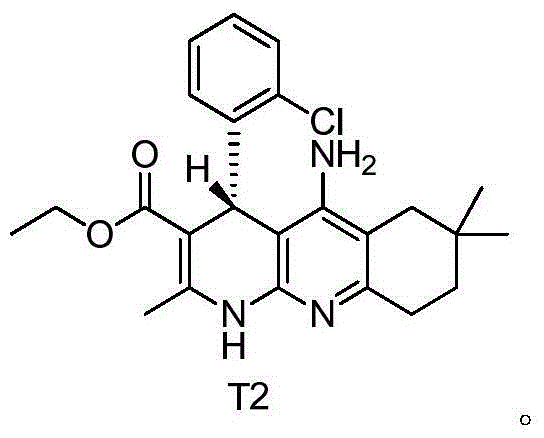
[0040] Example 2: Left-handed (S) 5-amino-4-(2-chlorophenyl)-2,7,7-trimethyl-1,4,6,7,8,9-hexahydrobenzo[b ][1,8]Naphthyridine-3-carboxylic acid ethyl ester (compound T1) and dextrorotatory (R) 5-amino-4-(2-chlorophenyl)-2,7,7-trimethyl-1 , Preparation of ethyl 4,6,7,8,9-hexahydrobenzo[b][1,8]naphthyridine-3-carboxylate (compound T2)
[0041]
[0042] Compound C was resolved to obtain compounds D1 and D2
[0043] Main equipment and chromatographic parameters: Waters company SFC-350 preparative chromatograph (equipped with Guangzhou Yanchuang ChiralCNOD preparative column, specification: 50×250mm, 5μm). Column temperature: 35°C, mobile phase: CO 2 / Ethanol=80 / 20, flow rate: 280g / min, cycle time: 2.5min, back pressure: 100Bar, detection wavelength: 214nm, sample concentration: 0.080g / mL, injection volume: 5mL (maximum sample load 500mg / time ).
[0044] Table 1 resolves the SFC chromatogram data of compound C
[0045] Optical isomers
retention time (min)
P...
Embodiment 3
[0056] Example 3: Single crystal preparation of compound T1 hydrochloride.
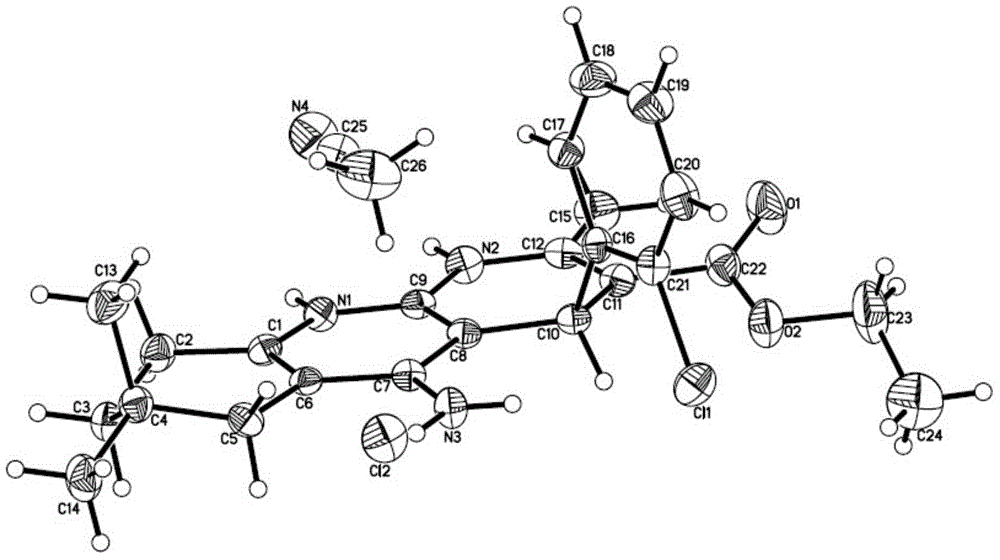
[0057] Take 9 mg of compound T1 hydrochloride, dissolve it in a mixed solvent of 400 μL of acetonitrile and 300 μL of dichloromethane, and place it at room temperature for natural volatilization and crystallization to obtain a single crystal, which is Form A. Molecular structure diagram such as figure 1 shown.
[0058] Molecular formula C 24 h 28 ClN 3 o 2 ·CH 3 CN·HCl, the crystal structure is orthorhombic, the space group P212121, and the unit cell parameters are: α=90°, β=90°, V=2621.1(9)A, F(000)=1064, Dc=1.276g / cm 3 .
[0059] Crystal X-single crystal diffraction data:
[0060] loop_
[0061] _atom_site_label
[0062] _atom_site_type_symbol
[0063] _atom_site_fract_x
[0064] _atom_site_fract_y
[0065] _atom_site_fract_z
[0066] _atom_site_U_iso_or_equiv
[0067] _atom_site_adp_type
[0068] _atom_site_occupancy
[0069] _atom_site_symmetry_multiplicity
[0070] _atom_sit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com