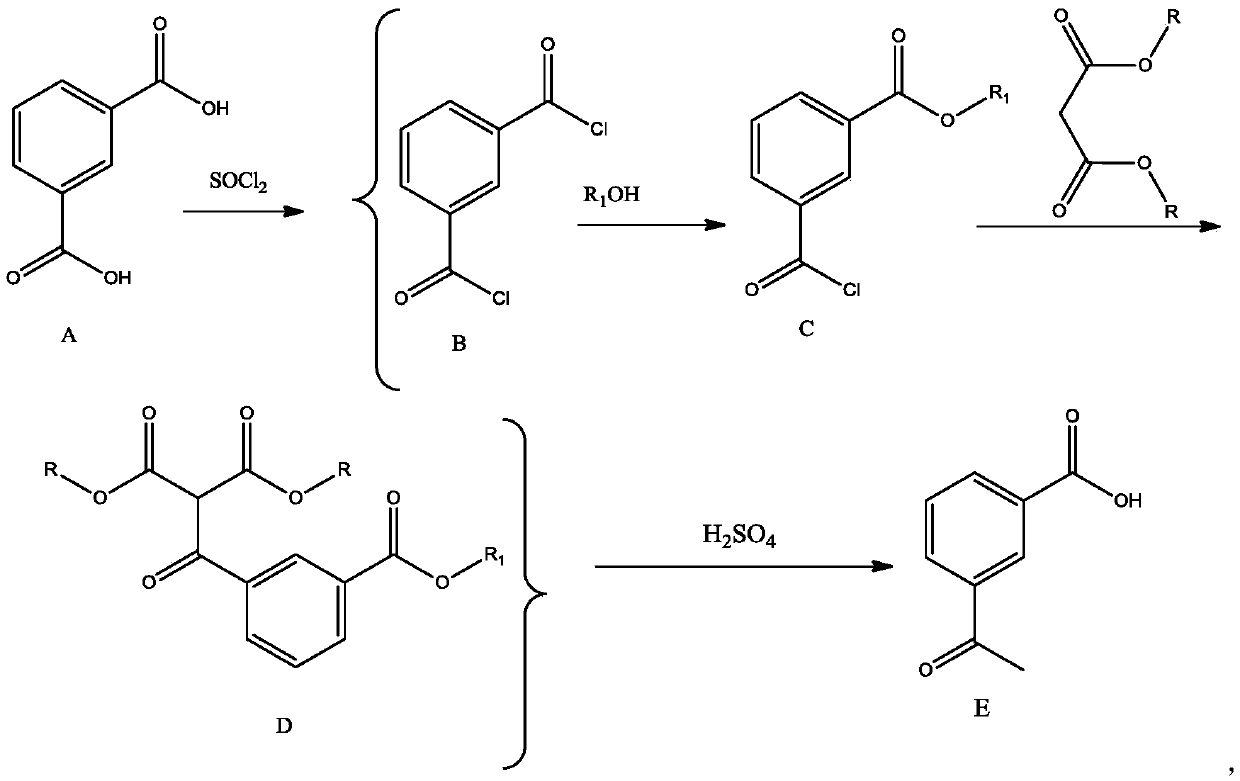
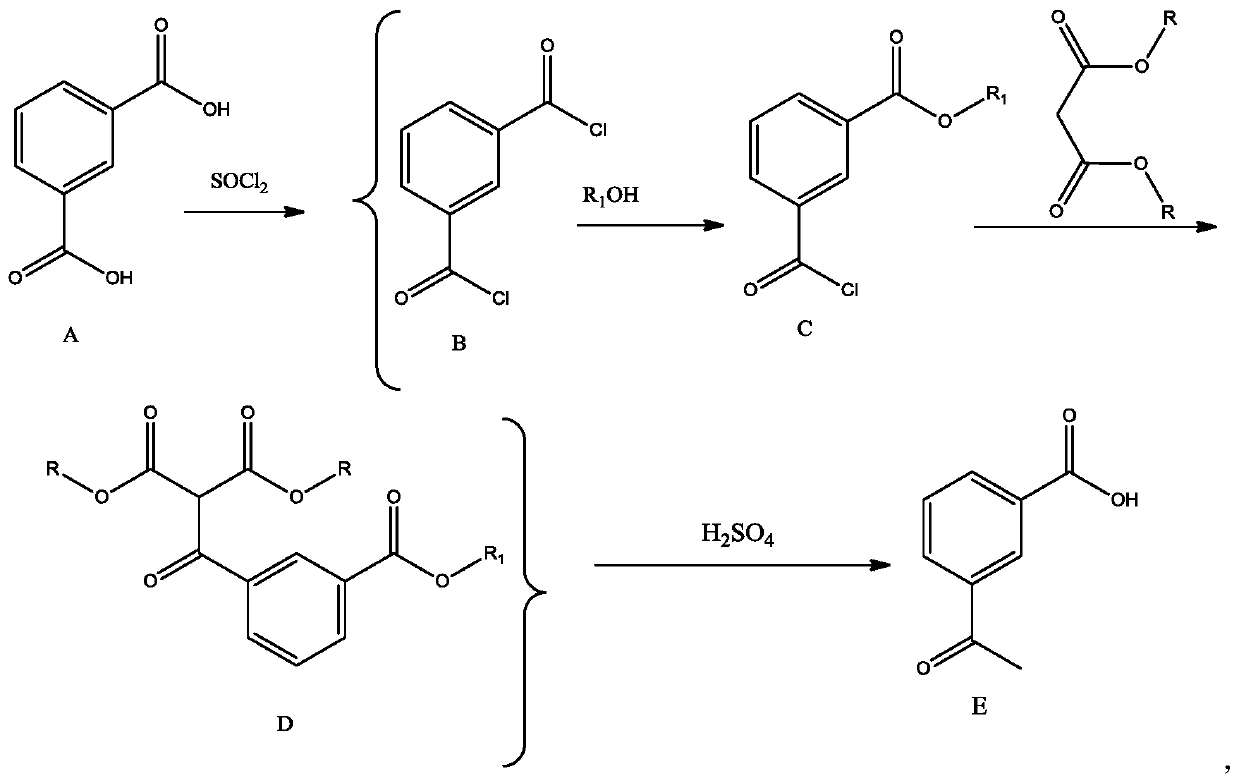
One-pot synthetic method for m-acetylbenzoic acid
A technology for the synthesis of acetylbenzoic acid and its synthesis method, which is applied in chemical instruments and methods, preparation of carboxylate, preparation of carboxylate, etc., can solve the problems of being difficult to be suitable for industrial production, potential safety hazards, unstable properties, etc., and achieve reduction in process Cost, mild reaction conditions, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 20g of isophthalic acid, 3 drops of DMF and 300ml of toluene to a 500ml three-necked reaction flask in sequence, raise the temperature to about 60°C, add 22ml of thionyl chloride dropwise, raise the temperature and reflux for 1 hour until no bubbles come out, steam the excess under normal pressure SOCl 2 and part of toluene (recovered and used mechanically), then cooled to room temperature, added 16.6ml triethylamine to the reaction bottle, stirred evenly, added dropwise 4.8ml methanol to the bottle, stirred for 1h after the dropwise addition, and TLC detected that the reaction was complete to obtain A solution of monomethyl formyl chloride. Add 33.4ml triethylamine, 14.4gCaCl successively under stirring 2 Powder and 22ml of diethyl malonate, stir at room temperature for 1 hour after the addition is complete, TLC detects that the reaction is complete, then add 50ml of 3mol / L hydrochloric acid to the reaction bottle to adjust the pH to about 1, separate the organic ...
Embodiment 2
[0034] Add 40g of isophthalic acid, 3 drops of DMF and 300ml of toluene to a 500ml three-necked reaction flask in sequence, raise the temperature to about 60°C, add 44ml of thionyl chloride dropwise, raise the temperature and reflux for 1 hour until no bubbles come out, steam the excess at normal pressure SOCl 2 and part of toluene (recovery for mechanical use), then cooled to room temperature, 33.4ml triethylamine was added in the reaction bottle, after stirring evenly, 9.5ml n-propanol was added dropwise in the bottle, and after the dropwise addition was completed and stirred for 1h, TLC detected that the reaction was complete, and obtained A solution of m-benzoyl chloride mono-n-propyl ester. Add 66.8ml triethylamine, 28.8g CaCl 2Powder and 44ml dimethyl malonate, stir at room temperature for 1 hour after the addition, TLC detects that the reaction is complete, then add 80ml of 3mol / L hydrochloric acid to the reaction bottle to adjust the pH to about 1, separate the organi...
Embodiment 3
[0036] Add 60g of isophthalic acid, 6 drops of DMF and 400ml of toluene to a 1000ml three-necked reaction flask in sequence, raise the temperature to about 60°C, add 66ml of thionyl chloride dropwise, raise the temperature and reflux for 1 hour until no bubbles come out, steam the excess under normal pressure SOCl 2 and part of toluene (recovered and used mechanically), then cooled to room temperature, 50ml of triethylamine was added to the reaction bottle, after stirring evenly, 14.4ml of methanol was added dropwise to the bottle, and the dropwise addition was completed and stirred for 1h. TLC detected that the reaction was complete, and m-benzylbenzene was obtained. A solution of monomethyl chloride. Add 90ml triethylamine, 90g CaBr successively under stirring 2 Powder and 22ml of diethyl malonate, stir at room temperature for 1 hour after the addition is complete, TLC detects that the reaction is complete, then add 150ml of 3mol / L hydrochloric acid to the reaction bottle t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap