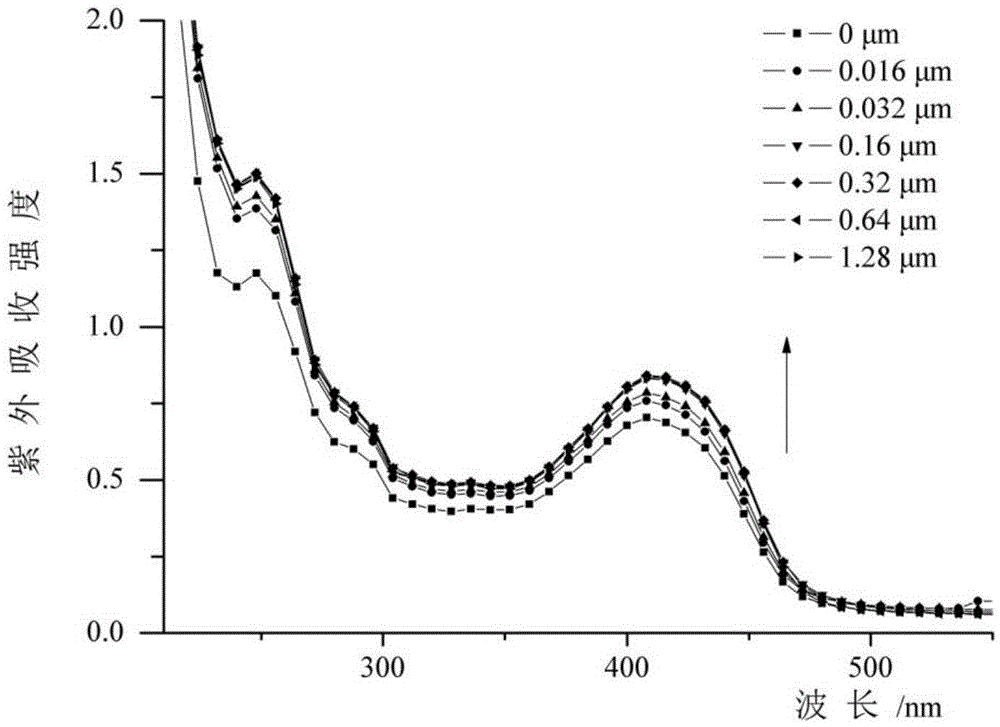
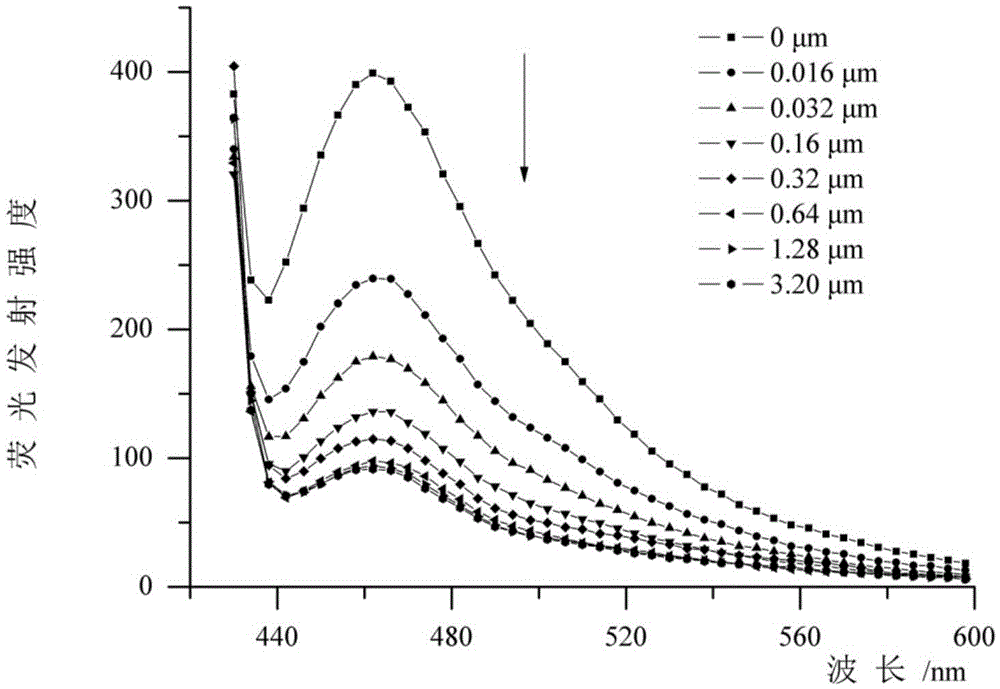
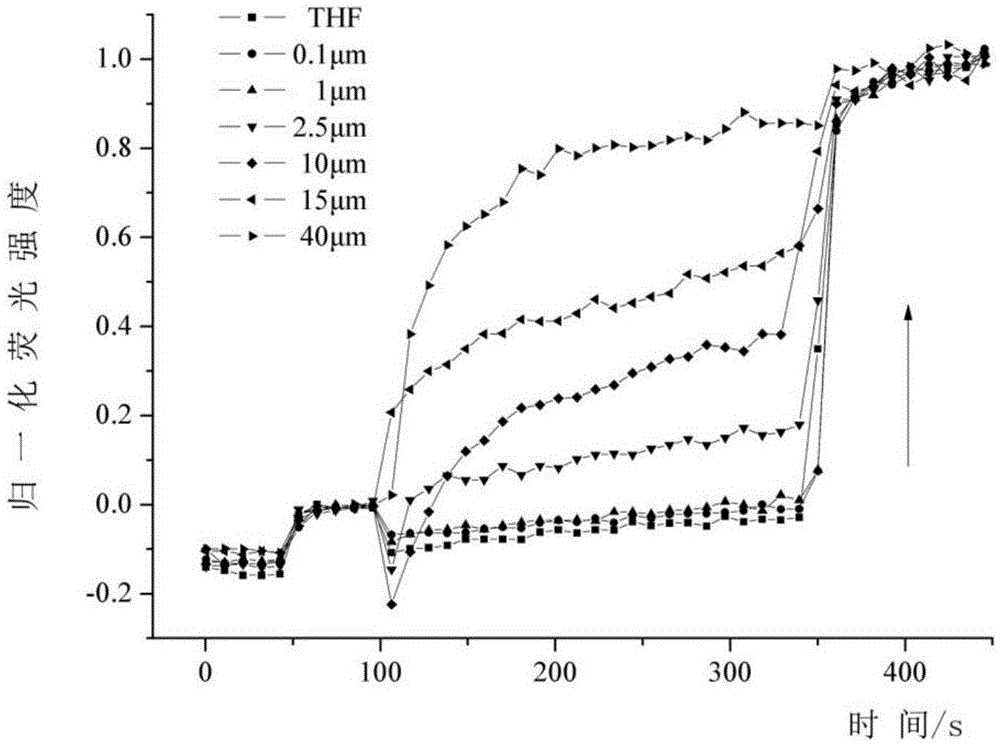
Preparation and application of asymmetric poly(phenyleneethynylene) oligomer with ion transmembrane transfer activity
A polyphenylene acetylene, asymmetric technology, applied in the field of artificial bionics, can solve the problems of limited application and low transmembrane activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) Weigh 10g of hydroquinone (90mmol) and 13.8g of potassium carbonate (99mmol) into a 250mL round bottom flask, measure about 100mL of anhydrous N,N-dimethylformamide into the reaction system, and heat to 90°C, after 30 minutes of reaction, a small amount of potassium iodide was added to accelerate the reaction process. Dissolve 16mL (90mmol) bromooctane in 20mL of anhydrous N,N-dimethylformamide and slowly add it dropwise to the reaction flask, and monitor by spotting. After the reaction was completed, the pH of the reaction system was adjusted to neutral, extracted twice with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, the solvent was removed by suction filtration, and purified by column chromatography (petroleum ether: ethyl acetate = 4:1 , v / v), 18 g of yellow solid 1 was obtained, yield 90%. 1 HNMR (CDCl 3 ,400MHz): δ=6.85–6.69(m,4H),3.89(t,J=6.6Hz,2H),1.81–1.67(m,2H),1.49–1.39(m,2H),1.37–1.24(m ,9H),0.88(t,J=6.9Hz,3H); 13 CNMR...
Embodiment 2
[0083] Step (1)-(10) embodiment 1 is identical, and step (11) is as follows:
[0084] (11) Synthesis of stereoregular oligomer P2:
[0085] Under anhydrous and oxygen-free conditions, take a 50mL drying tube and add compound 11 (0.06g, 0.076mmol), cuprous iodide (0.0017g, 0.0076mmol), tetrakistriphenylphosphine palladium (0.0106g, 0.0076mmol) in sequence, and pump Under argon atmosphere after vacuum, toluene (2.5 mL), triethylamine (2.5 mL) and tetrahydrofuran (2.5 mL) were injected by syringe. Freeze to remove oxygen, react at room temperature for 24 hours, the solution gradually turns bright yellow, and the upper layer shows strong green fluorescence, heat up to 60°C and react for about 72 hours; when the reaction is completed, pour into saturated sodium chloride aqueous solution to quench the reaction, two Methyl chloride was extracted twice, dried over anhydrous magnesium sulfate, and the solvent was removed by suction filtration. The resulting bright yellow solid was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com