Method for increasing yield of L-alanyl-L-glutamine from recombinant escherichia coli
A glutamine and alanyl technology, applied in microorganism-based methods, recombinant DNA technology, biochemical equipment and methods, etc., can solve the problem of affecting cell growth and metabolism, low substrate conversion rate, and instability of proglutamate dipeptide production strains. and other problems, to achieve the effect of improving enzyme catalytic efficiency, broad industrial application prospects, and improving substrate conversion rate and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Acquisition of genes encoding multidrug efflux transporters
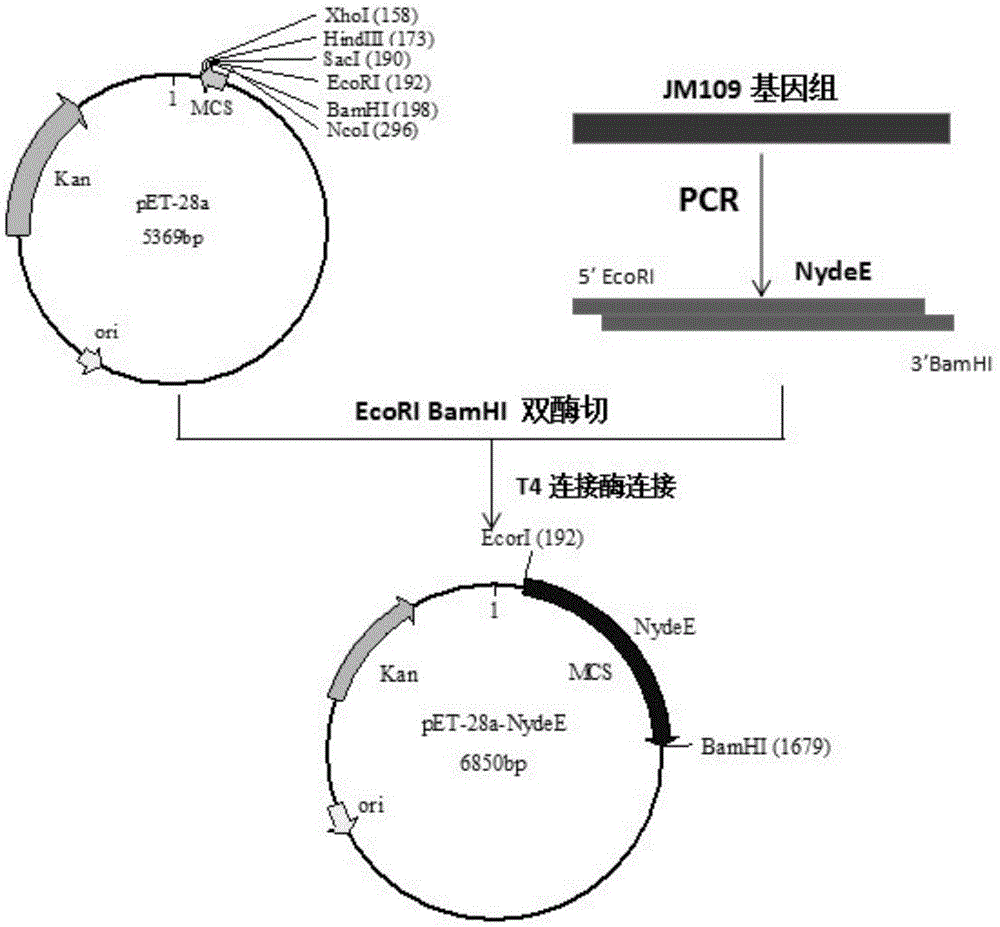
[0036] Multidrug efflux transporter (ydeE) can specifically and effectively transport L-alanyl-L-glutamine from intracellular to extracellular, and a ydeE gene sequence (GeneID: 946083) published in NCBI was designed to For primer P1 (TTT GAATTC TTTAGCCCCCATACGACCAC), P2(TTT GGATCCTCAACAAAGCGCGGGCTGCC), using Escherichia coli genome as a template to carry out PCR amplification to obtain the target gene ydeE and its own promoter. The target gene ydeE and its own promoter were named NydeE. The P1 primer contains an EcorI restriction site, and the P2 primer contains a BamHI restriction site, which is convenient for subsequent restriction restriction and construction of vectors. The Escherichia coli genome was obtained through a bacterial genome DNA extraction kit purchased from Beijing Tiangen.
Embodiment 2
[0037] Embodiment 2: Obtaining of recombinant plasmid pET28a-NydeE containing NydeE
[0038] The NydeE target fragment obtained in Example 1 and the pET28a plasmid were simultaneously digested with EcorI and BamHI. The double enzyme digestion system is as follows:
[0039]
[0040] After 3 hours of double enzyme digestion, electrophoresis, gel cutting to recover the target fragment, T4 ligase ligation at 16°C for 12-16 hours. The connection system is as follows:
[0041]
[0042] After overnight ligation at 16°C, 3 μL of the ligation solution was transformed into Escherichia coli JM109 competent cells. The transformation process is as follows: add 3 μL of connection solution to 50 μL of JM109 competent cells, then add 10 μL of 5×KCM buffer, mix well, pre-cool on ice for 30 minutes, heat shock at 42°C for 90 seconds, and place on ice After standing still for 5 minutes, add 500 μL of LB medium, recover at 37°C, 220 rpm for 60 minutes. After resuscitation, take an appro...
Embodiment 3
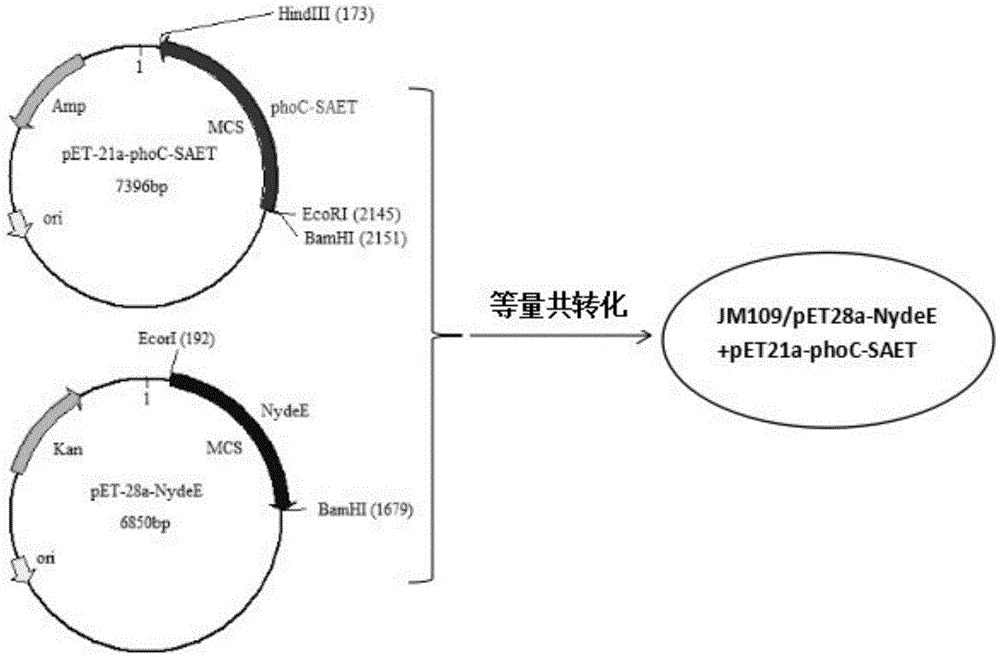
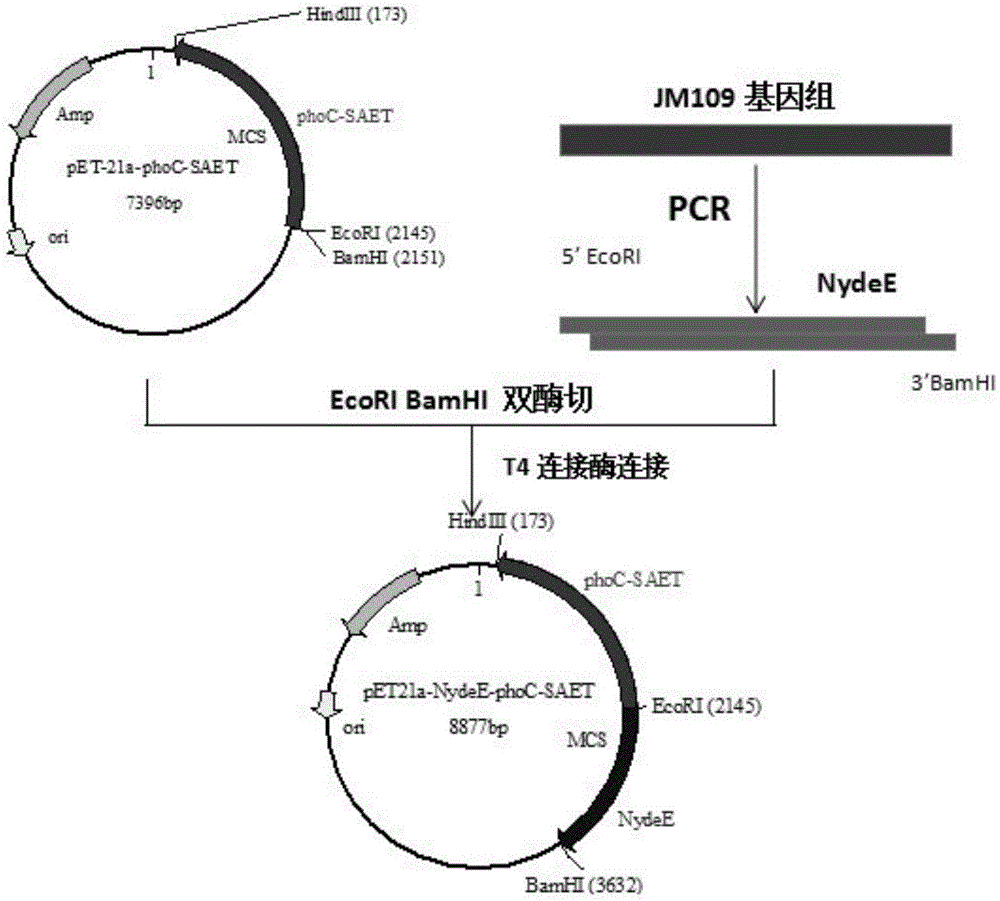
[0043] Embodiment 3: Obtaining of recombinant plasmid pET21a-phoC-SAET containing SAET
[0044] The strain JM109 / pET21a-phoC-SAET containing the target plasmid stored in the laboratory was activated and cultured at 37°C and 220rpm for 12-16 hours. The cultured bacterial liquid was taken to extract the plasmid, and all operations were carried out in strict accordance with the instructions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com