Two-photon deep red emission fluorescent probe for imaging cell membranes in tissues based on molecular rotors
A fluorescent probe and two-photon technology, applied in the direction of fluorescence/phosphorescence, luminescent materials, analytical materials, etc., can solve the problems of increasing the difficulty of synthesis and limiting applications, and achieve large Stokes shift, good compatibility, The effect of filling the gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of N,N-Diformylaniline
[0028] Dry DMF (3.7mL) and phosphorus oxychloride (3.7mL, 40.7mmol) were mixed into a three-necked flask and stirred at 0°C. After 30 min, triphenylamine (1.0 g, 4.0 mmol) dissolved in chloroform was added dropwise to the above mixture, heated and stirred for 24 h. Cool to room temperature after the reaction, add appropriate amount of sodium hydroxide and water, then extract with dichloromethane and wash with water. Dry with anhydrous sodium sulfate. Finally, a mixture of petroleum ether and ethyl acetate was used for column chromatography to obtain the final product.
[0029] 1 HNMR (400MHz, DMSO-d6): δ (ppm) 9.88 (s, 2H), 7.85 (d, J = 8.64Hz, 4H), 7.49 (t, J = 7.84Hz, 2H), 7.33 (t, J = 7.4Hz, 1H), 7.22(d, J=8.4Hz, 2H), 7.17(d, J=8.56Hz, 4H).
[0030] Synthesis of 1-dodecyl-4-picoline-1 iodide
[0031] Mix 4-methylpyridine (2 mL, 20 mmol) and 1-iodododecane, use ethanol as solvent, and heat to reflux. After 24 hours, the reaction...
Embodiment 2
[0036] Example 2: Immortalized cancer cell (HeLa and SiHa) and normal cell (HUVEC) culture
[0037] HeLa, SiHa, and HUVEC cell lines were all stored at 37°C, 5% CO 2 CO 2 cultured in an incubator. HeLa and SiHa cell lines were adherently cultured in H-DMEM medium containing 10% fetal bovine serum and 1% double antibody. HUVEC cell lines were adhered to culture medium containing 10% fetal bovine serum and 2ng / mL FGF-2M199. When the cells grow to the logarithmic phase, culture the slices: ① Soak the coverslips in absolute ethanol for 30 minutes, dry them with an alcohol lamp, and place them in a disposable 35mm culture dish; ② Wash the cells in the 100mL cell bottle with PBS. Three times, digest with 1mL 0.25% trypsin for 3-5 minutes, pour out the medium carefully, add a small amount of fresh medium and pipette evenly, after counting the cells, leave cells with a suitable density, and add the medium to the required volume (The final concentration of control cells was 1×10 5...
Embodiment 3
[0038] Example 3: Two-photon fluorescence microscopy experiment of T1 stained HeLa cells
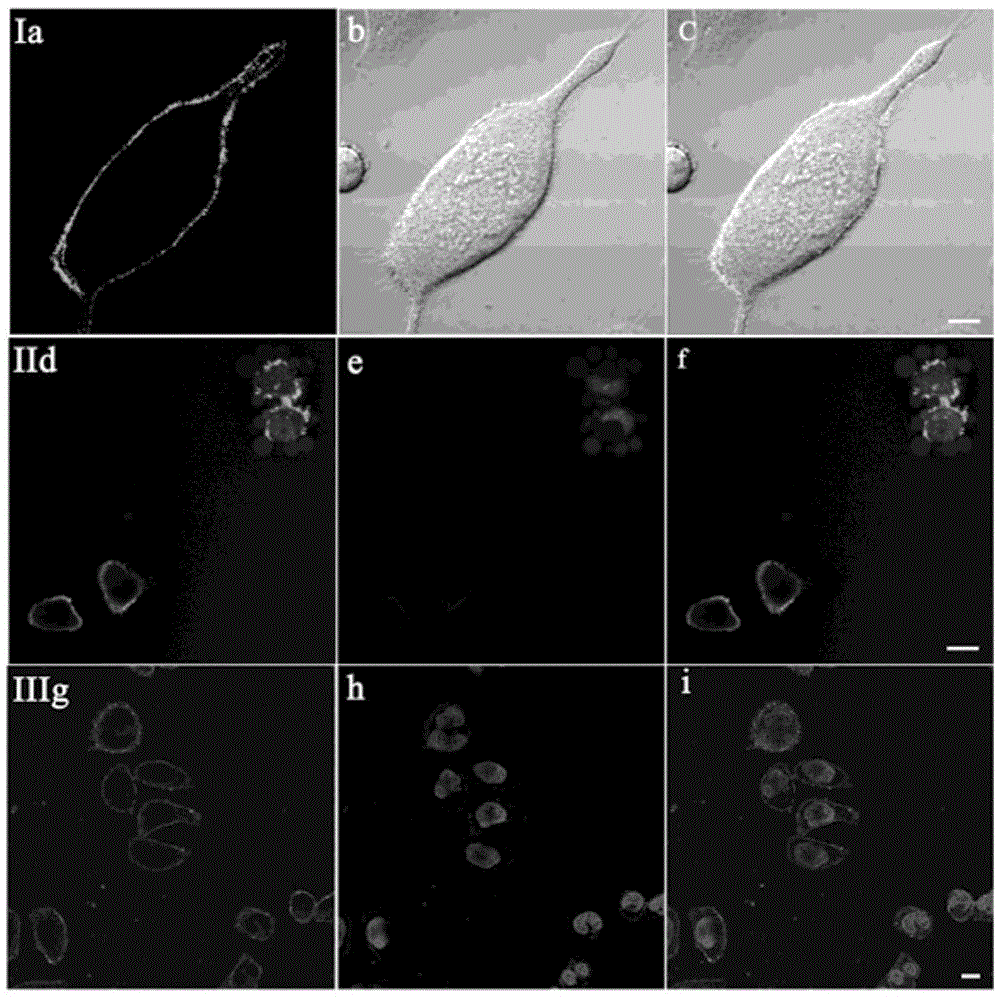
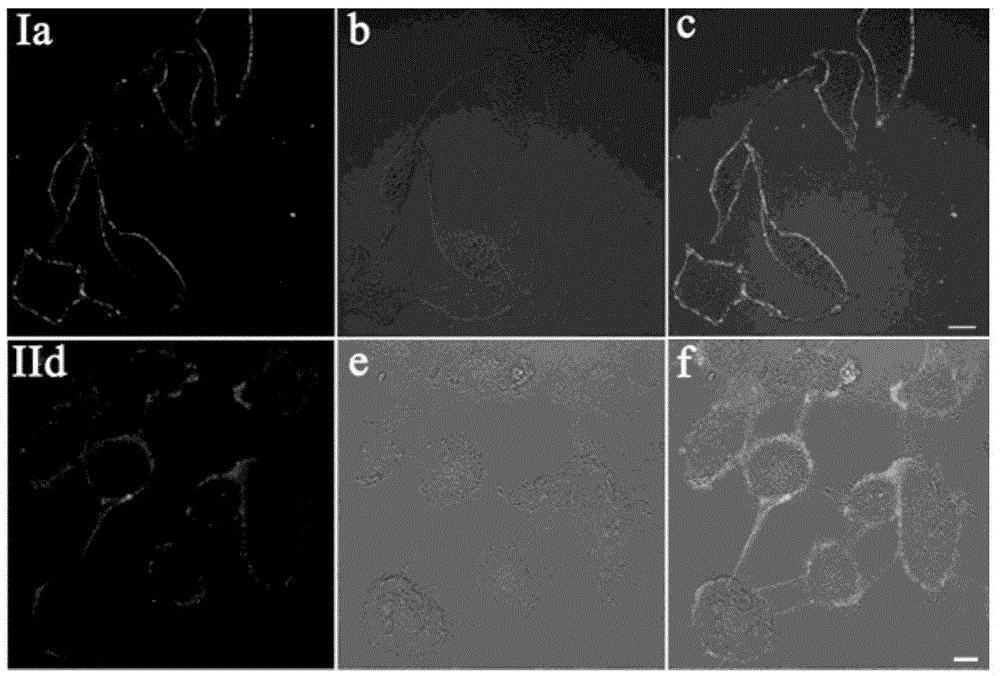
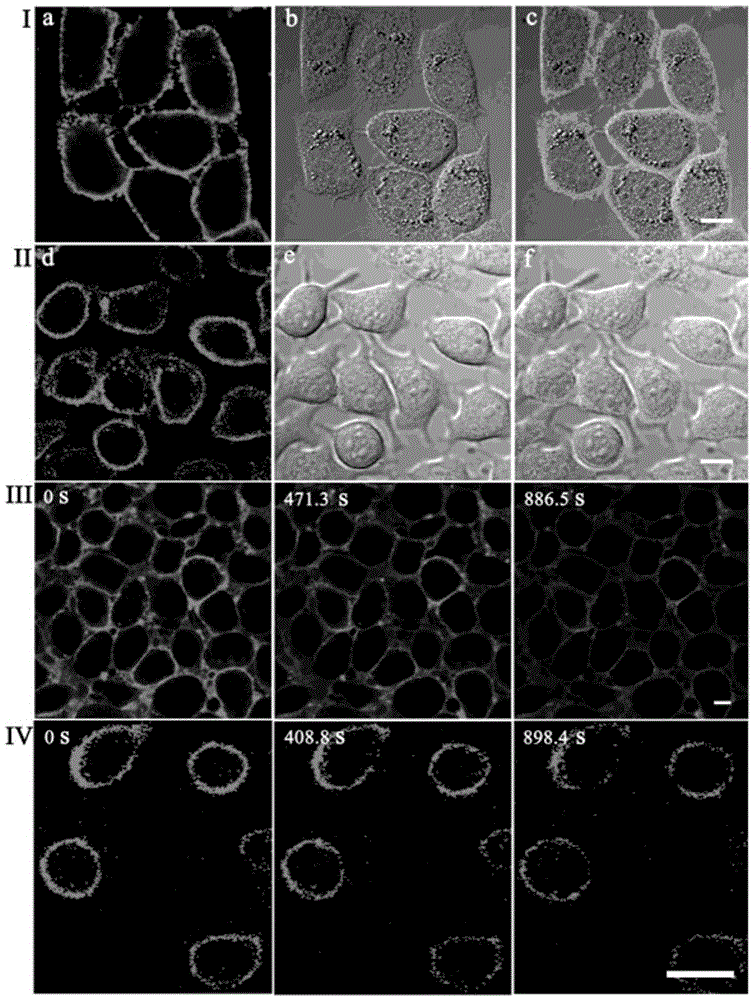
[0039] Stain the attached cell slides with 2 μM T1, and in CO 2 Incubate in the incubator for 20min. After aspirating the culture medium, wash it three times with PBS, take out the cell slide, put the cell growth side down on the glass slide, observe under the two-photon fluorescence microscope, and find that the cell membrane is evenly and continuously stained by T1. Therefore, the probe T1 of the present invention is a two-photon cell membrane probe.
[0040] see results figure 1 (I). Two-photon photographs obtained under 800 nm laser irradiation after staining with T1. Among them, picture a is a two-photon fluorescence photo; picture b is a differential interference picture of bright-field laser scanning; picture c is a superimposed picture of a and b.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com