Type-1 obeticholic acid preparation method
A technology for obeticholic acid and preparation process, which is applied in the field of preparation of obeticholic acid type 1, can solve problems such as unfavorable industrialized production, increased operation steps, prolonged production cycle, etc., and achieves shortening production steps, short production cycle, and simplification. effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
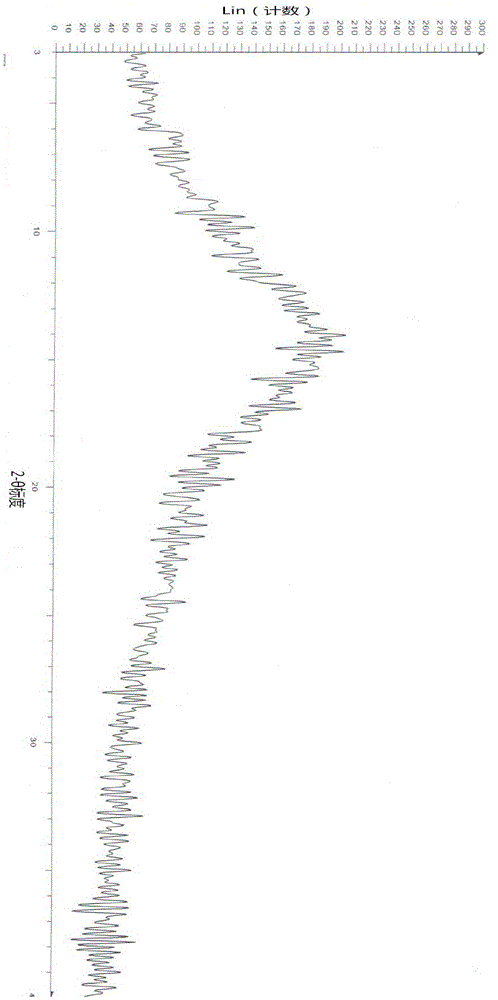
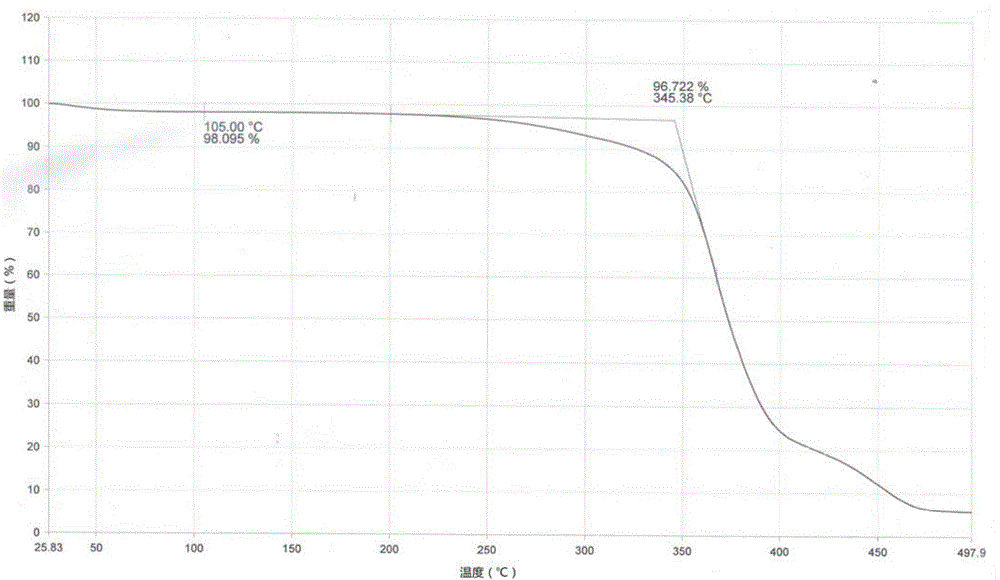
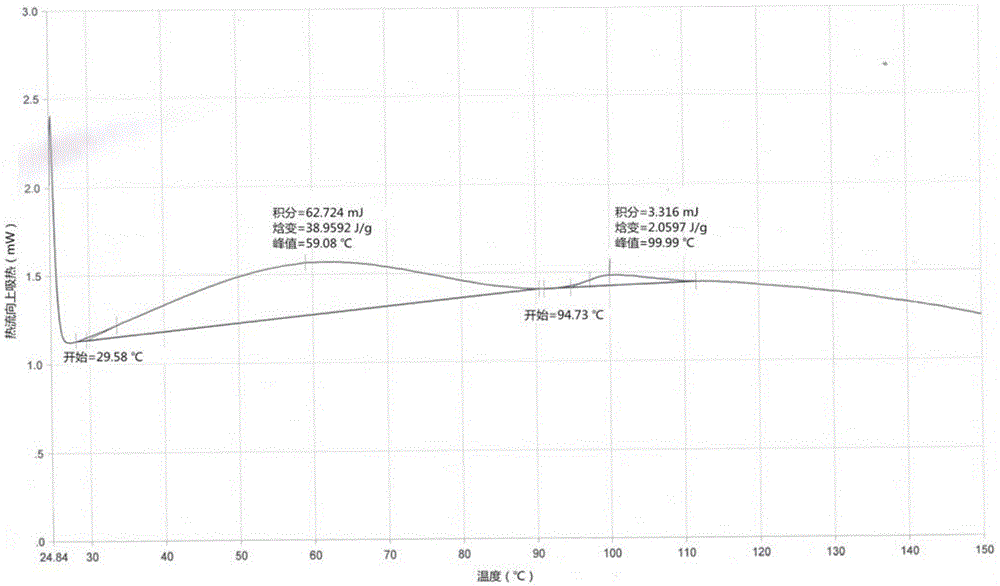
Image
Examples
Embodiment 1
[0051] Add 3α-hydroxyl-6α-ethyl-7-keto-5β-cholan-24-acid (50g, 0.12mol), sodium hydroxide (24g, 0.60mol) and water (400mL) in the reaction flask, stir The temperature was raised to 95° C., sodium borohydride (9.1 g, 0.24 mol) was added, and the mixture was reacted for 5 hours. TLC traced that the reaction of the raw materials was complete. After the reaction, cool to 40°C, add ethyl acetate (400mL) and water (200mL), adjust the pH to 3 with 37% hydrochloric acid, separate the layers, dry the organic phase over anhydrous sodium sulfate for 2 hours, filter, and reduce the filtrate at 40°C Evaporated to dryness under pressure to obtain a colorless oily residue. Add water (750mL) and NaOH (9.6g, 0.24mol) to the residue and heat up to 40°C, stir until dissolved, slowly add 0.4mol / L dilute hydrochloric acid solution dropwise to adjust the pH to 3.0, continue to keep stirring for 30min, and cool to 10°C Stir and crystallize for 2 hours, filter, and vacuum-dry the filter cake at 50° ...
Embodiment 2
[0057]Add 3α-hydroxy-6α-ethyl-7-keto-5β-cholan-24-oic acid (50g, 0.12mol), sodium methoxide (38.9g, 0.72mol), methanol (100mL) and water ( 300 mL), heated to 80°C with stirring, added sodium borohydride (9.1 g, 0.24 mol), and reacted for 7 hours. TLC traced that the reaction of the raw materials was complete. After the reaction, cool to 40°C, add dichloromethane (400mL) and water (200mL), adjust the pH to 4 with 85% phosphoric acid, separate the layers, dry the organic phase over anhydrous sodium sulfate for 2 hours, filter, and reduce the filtrate at 40°C Evaporated to dryness under pressure to obtain a pale yellow oily residue. Add water (750mL) and sodium methoxide (19.4g, 0.36mol) to the residue and heat up to 30°C, stir until dissolved, slowly add 0.2mol / L dilute hydrochloric acid solution dropwise to adjust the pH to 4.0, continue to keep stirring for 30min, and cool to 15 Stir and crystallize at ℃ for 3 hours, filter, and vacuum-dry the filter cake at 40℃ for 5 hours t...
Embodiment 3
[0059] Add 3α-hydroxyl-6α-ethyl-7-ketone-5β-cholane-24-acid ethyl ester (54g, 0.12mol), NaOH (24g, 0.60mol), ethanol (230mL), water ( 200 mL), heated to 90°C with stirring, added potassium borohydride (12.9 g, 0.24 mol), and reacted for 6 hours, TLC traced the complete reaction of raw materials. After the reaction, cool to 40°C, add toluene (400mL) and water (200mL), adjust pH to 4 with 37% hydrochloric acid, separate liquids, dry the organic phase over anhydrous sodium sulfate for 2 hours, filter, and evaporate the filtrate at 60°C under reduced pressure Dry to a colorless oily residue. Add water (750mL) and NaOH (9.6g, 0.24mol) to the residue and heat up to 40°C, stir until dissolved, slowly add 0.2mol / L dilute hydrochloric acid solution dropwise to adjust the pH to 3.0, continue to keep stirring for 30min, and cool to 10°C Stir and crystallize for 2 hours, filter, and vacuum-dry the filter cake at 50° C. for 3 hours to obtain a white solid, obeticholic acid type 1 (41.9 g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com