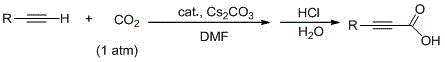Method for preparing propiolic acid compounds
A compound, the technology of propiolic acid, which is applied in the field of preparation of propiolic acid compounds, can solve the problems of expensive metal-organic reagents, expensive rare earth compounds, and little practical application significance, and achieve air stability, which is conducive to large-scale Industrial application, the effect of a single component
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
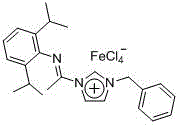
[0020] Embodiment one: the ionic iron (III) complex (molecular formula is [{(RNC(CH 3 ))NCHCHN(CH 2 Ph)}CH][FeCl 4 ] (R is 2,6-diisopropylphenyl)) synthesis
[0021] [{(RNC(CH 3 ))NCHCHN(CH 2 Ph)}CH]Cl (R is 2,6-diisopropylphenyl) (0.40 g, 1.0 mmol) was added to anhydrous ferric chloride (0.16 g, 1.0 mmol) in tetrahydrofuran solution, 25 React at ℃ for 3 hours, remove the solvent in vacuo, wash with hexane, drain, extract with tetrahydrofuran, centrifuge, transfer the clear liquid, add hexane to the clear liquid for recrystallization, and precipitate yellow crystals at room temperature with a yield of 93%.
[0022] The product was subjected to elemental analysis, and the results are shown in Table 1.
[0023] Table 1 Elemental analysis results of ionic iron(III) complexes containing monoimine functionalized imidazolium cations
[0024]
C:(%) H:(%) N:(%) theoretical value 51.64 5.42 7.53 actual value 51.70 5.38 7.66
[0025] Since the i...
Embodiment 2
[0029] Embodiment 2: [{(RNC(CH 3 ))NCHCHN(CH 2 Ph)}CH][FeCl 4 ](R is 2,6-diisopropylphenyl) catalyzed carboxylation reaction of phenylacetylene with carbon dioxide
[0030] Add catalyst (14.0mg, 0.025mmol, 5mol%), cesium carbonate (32.6mg, 1.0mmol), phenylacetylene (55μl, 0.5mmol), N , N -Dimethylformamide (3 ml), fed with carbon dioxide, reacted at 70°C under normal pressure for 18 hours. The reaction was cooled to room temperature, diluted with water, acidified with hydrochloric acid, extracted with ether, the ether layer was washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and the solvent was removed in vacuo to obtain the product with a yield of 97%.
[0031] Dissolve the product in CDCl 3 Medium (about 0.4mL), seal the tube, measure and characterize on a UnityInova-400 NMR instrument at room temperature: 1 HNMR (400MHz, CDCl 3 ,TMS):δ7.63-7.61(m,2H,Ar- H ),7.51-7.47(m,1H,Ar- H ),7.42-7.38(m,2H,Ar- H )ppm.
Embodiment 3
[0032] Embodiment three: [{(RNC(CH 3 ))NCHCHN(CH 2 Ph)}CH][FeCl 4 ](R is 2,6-diisopropylphenyl) catalyzed carboxylation reaction of 4-methylphenylacetylene with carbon dioxide
[0033] Add catalyst (14.0 mg, 0.025 mmol, 5 mol%), cesium carbonate (32.6 mg, 1.0 mmol), 4-methylphenylacetylene (63 μl, 0.5 mmol) successively into the reaction flask, N , N -Dimethylformamide (3 ml), fed with carbon dioxide, reacted at 65°C under normal pressure for 18 hours. The reaction was cooled to room temperature, diluted with water, acidified with hydrochloric acid, extracted with ether, the ether layer was washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and the solvent was removed in vacuo to obtain the product with a yield of 90%.
[0034] The product was dissolved in DMSO- d 6 Medium (about 0.4mL), seal the tube, measure and characterize on a UnityInova-400 NMR instrument at room temperature: 1 HNMR (400MHz, DMSO- d 6 ,TMS):δ7.52(d, J =8.0Hz,2H,Ar- H ),7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com