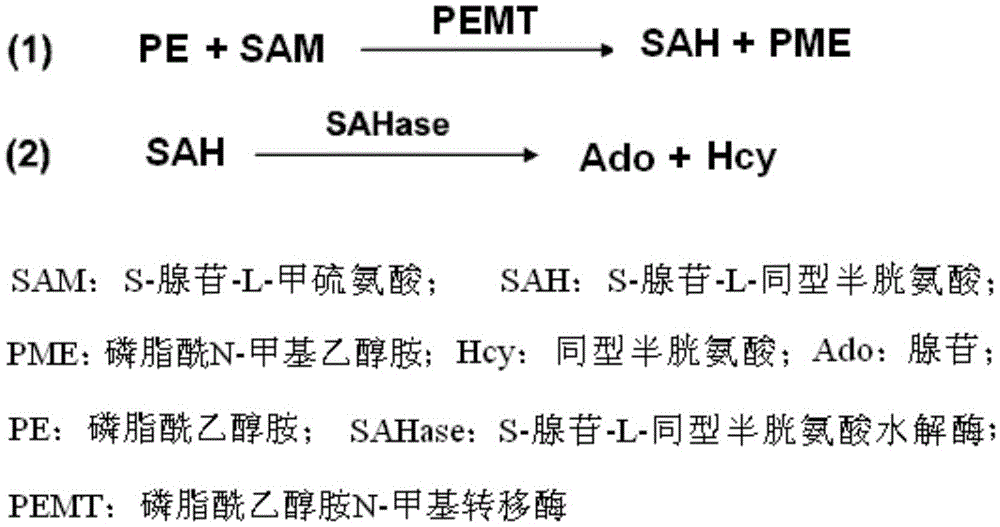
Phosphatidyl ethanolamine N-methyltransferase activity detecting method
A technology of phosphatidylethanolamine and methyltransferase, which is applied in the fields of biomedicine, clinical diagnosis and detection, and can solve the problems that the detection method of enzyme activity is not easy to popularize.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Crude Extraction of Rat Liver Endoplasmic Reticulum
[0087] Weigh 20 g of rat liver tissue from which adipose tissue has been excised, add 200 mL of 0.25 M (containing 0.01 M, pH 8 phosphate buffer) sucrose solution, and homogenize 20 times with a glass homogenizer. Sigma3-30K centrifuge at 2600 Centrifuge at 2800 rpm for 10 minutes, filter through four layers of gauze, and discard the precipitate. The supernatant was centrifuged at 15,000rpm for 30 minutes, and the resulting precipitate was the part containing the endoplasmic reticulum, which was suspended in 0.25M (containing 0.01M, pH8.0 phosphate buffer) sucrose solution, and the endoplasmic reticulum could be stored at -80°C Save it.
Embodiment 2
[0088] Example 2: Determination of phosphatidylethanolamine N-methyltransferase enzyme activity in rat liver endoplasmic reticulum (pre-treatment)
[0089] The PEMT detection reagent of the present embodiment is three doses of reagents, comprising
[0090] Reagent 1
[0091] Tris-hydrochloric acid buffer (pH8.5) 30mmol / L
[0092] SAM0.1mmol / L
[0093] Reagent 2
[0094] Tris-hydrochloric acid buffer (pH8.5) 30mmol / L
[0095] PE0.2mmol / L
[0096] Reagent 3
[0097] Potassium phosphate buffer (pH8.0) 30mmol / L
[0098] S-adenosyl-L-homocysteine hydrolase 10KU / L
[0099] The PEMT sample to be tested is the extracted endoplasmic reticulum of rat liver tissue. After crushing, add sodium cholate solution to the sample to make the final volume percentage concentration reach 0.5%. After mixing gently, place it on ice for 30 minutes to wait. use. The total protein dosage in the reaction system is 10ug, and the reaction system is 250ul. A total of 5 parallel samples were ma...
Embodiment 3
[0106] Embodiment 3: Determination of enzyme activity of phosphatidylethanolamine N-methyltransferase in rat liver endoplasmic reticulum (sample is processed in the early stage comparison)
[0107] The phosphatidylethanolamine N-methyltransferase detection reagent of this embodiment is three reagents, including
[0108] Reagent 1
[0109] Tris-hydrochloric acid buffer (pH8.5) 30mmol / L
[0110] SAM0.1mmol / L
[0111] Reagent 2
[0112] Tris-hydrochloric acid buffer (pH8.5) 30mmol / L
[0113] PE0.2mmol / L
[0114] Reagent 3
[0115] Potassium phosphate buffer (pH8.0) 30mmol / L
[0116] S-adenosyl-L-homocysteine hydrolase 10KU / L
[0117] The sample of phosphatidylethanolamine N-methyltransferase is the extracted endoplasmic reticulum of rat liver tissue. %, after mixing gently, place it on ice for 30min; add the same amount of sample suspension to another part, mix gently and place it on ice for 30min, the total protein dosage in the reaction system is 10ug, and the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com