Preparation method of tinib drug alhumin nano preparation used for injection
A technology of albumin nanometer and albumin nanoparticle, which is applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., can solve the problems of large amount of phospholipid, difficult industrialization of preparation process, poor stability, etc., and achieves reduction of dosage. , the effect of increasing stability and application security, and expanding the range of choices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: The parameter of high-pressure homogenization is for the influence of preparation albumin nanoparticle particle size
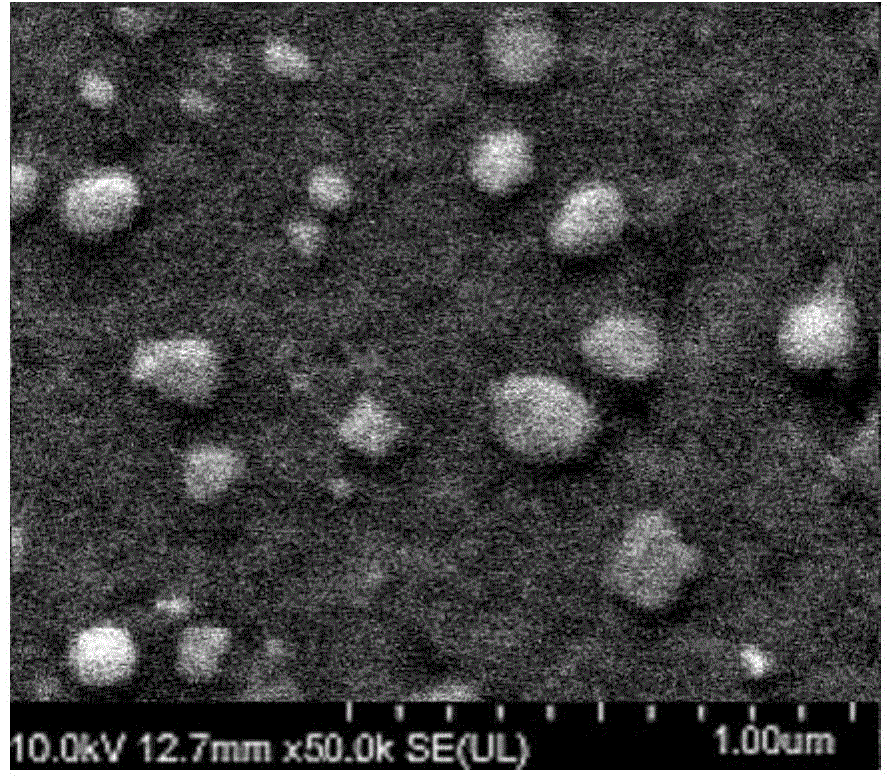
[0043] Weigh a certain amount of icotinib and polyoxyethylene castor oil and dissolve it in a mixed solvent of chloroform and ethanol as the oil phase, add it to the aqueous solution of human serum albumin, and control the volume ratio of the oil phase and the water phase to 1 :20~1:80, the mass ratio of icotinib to albumin is 1:5~1:10. Utilize high-shear dispersing emulsifier to emulsify (rotating speed: 5000r / min, shearing time: 10min) to form colostrum, quickly transfer to high-pressure homogenizer, homogenize according to the conditions in Table 1, and the obtained nanoemulsion is removed by thin film evaporation Organic solvent, that is, albumin nanoparticle suspension loaded with icotinib.
[0044] It can be seen from Table 1 that when the high-pressure homogenization pressure is increased from 5000psi to 15000psi, the particle size...
Embodiment 2
[0047] Example 2: Comparison of the amount of phospholipids required for the preparation of nanoparticles by the method of the present invention and the method of patent 2012101304132
[0048] Dissolve sorafenib and hydrogenated soybean lecithin in different proportions in dichloromethane as the oil phase, add human serum albumin aqueous solution with a pH of 4 to 7, and control the volume ratio of the oil / water phase to 1:50 to 1:100 , the mass ratio of sorafenib to albumin is 1:5-1:12. Sorafenib-loaded albumin nanoparticles were prepared by the method of the present invention and the method of patent 2012101304132 respectively, and the particle size was measured. The results are shown in Table 2.
[0049] The impact of the amount of hydrogenated soybean lecithin on the particle size of nanoparticles in the prescription of table 2
[0050]
[0051]
[0052] It can be seen that when the amount of hydrogenated soybean lecithin is used as a solubilizer, the particle size ...
Embodiment 3
[0053] Example 3: Comparison of the stability of nanoparticles prepared by the method of the present invention and the method of patent 2012101304132
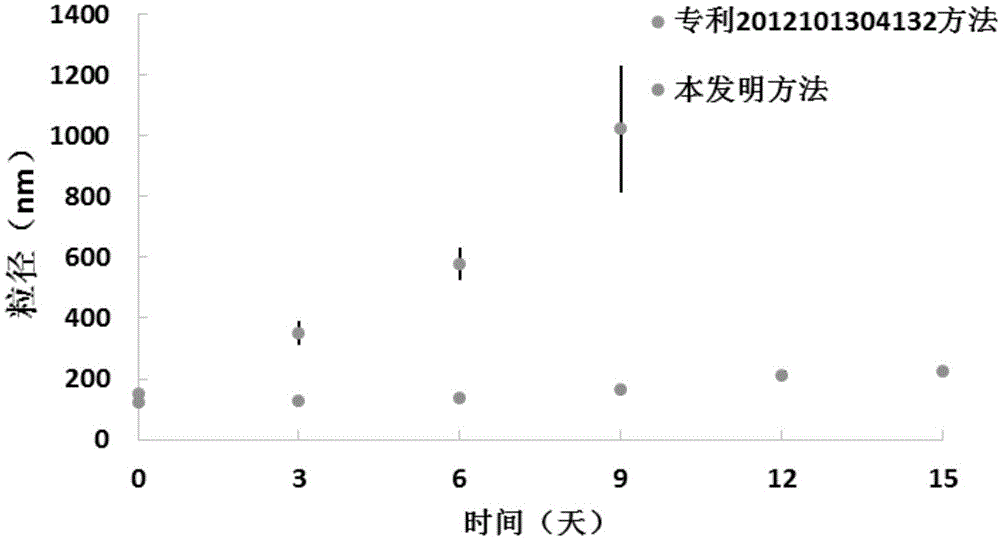
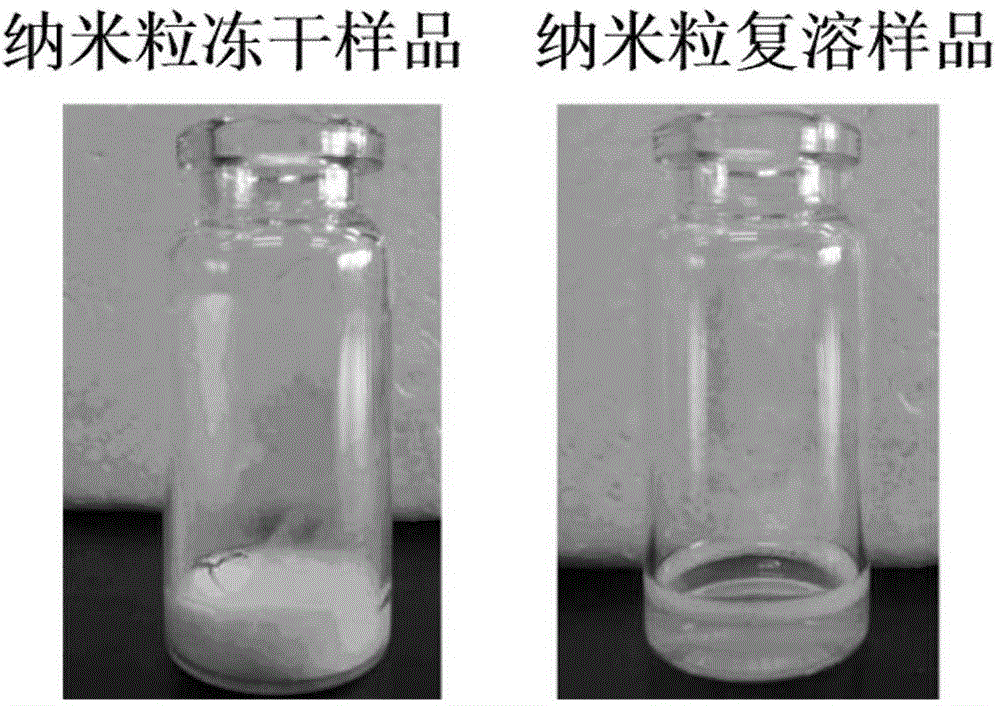
[0054] The albumin nanoparticles loaded with icotinib in Example 1 were prepared by the method of the present invention and the method of patent 2012101304132 respectively. Place the two suspensions of icotinib albumin nanoparticles at room temperature, observe their appearance and measure the particle size at 0, 3, 6, 9, 12, and 15 days respectively (attached figure 1 ). As a result, the icotinib-loaded albumin nanoparticle suspension prepared by the method of patent 2012101304132 showed obvious drug precipitation on the 3rd to 6th day, and the particle size reached the μm level. However, the nanoparticle suspension prepared by the method of the present invention has better stability, no obvious suspension layering or precipitation was observed within 15 days, and the particle diameter of the nanoparticle slightly increased t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com