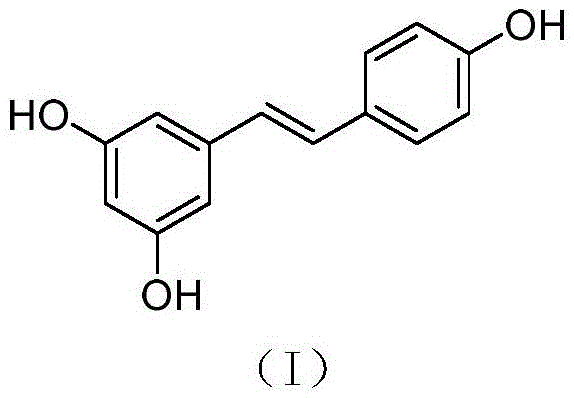
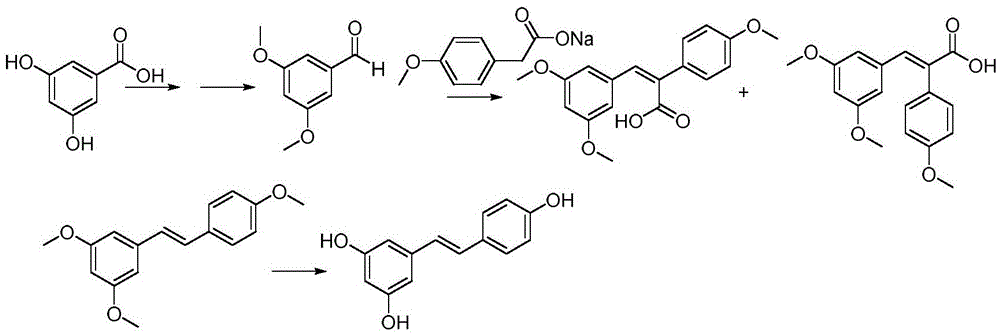
Synthesis method of resveratrol
A technology of resveratrol and dihydroxybenzyl alcohol, which is applied in the chemical industry, can solve the problems of high cost, complex process, and difficult separation, and achieve the effects of low cost, simple operation, and good reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
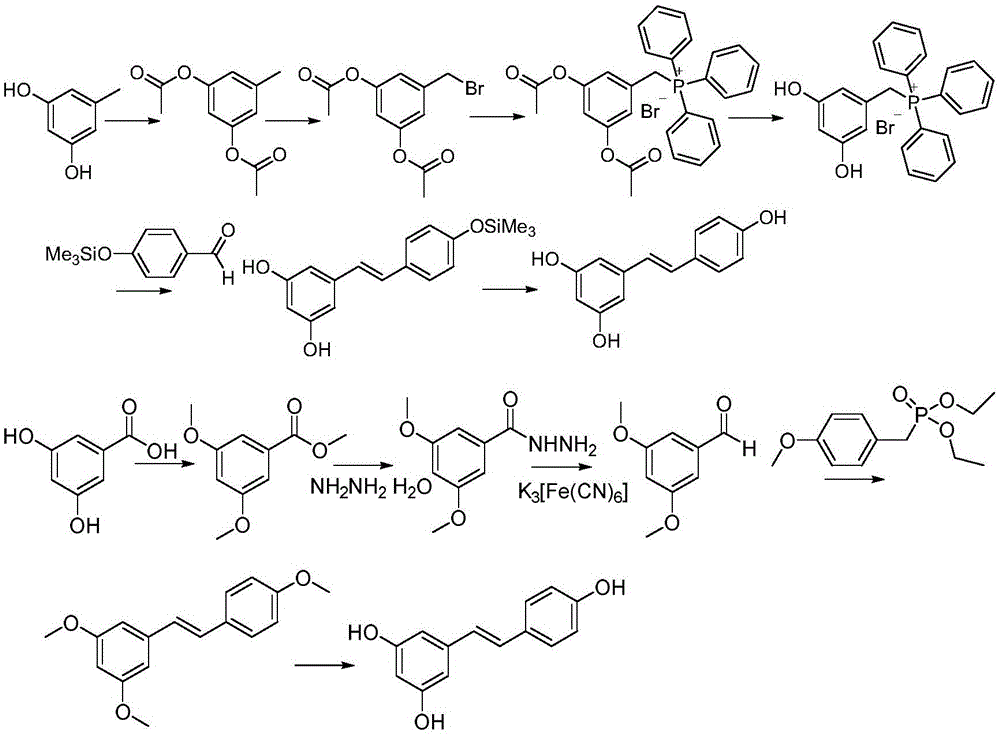
[0034] This reaction should be carried out under the protection of nitrogen, and try to keep the reaction system anhydrous. Add 5 g of sodium borohydride (132 mmol) and 54.5 g of ethylene glycol dimethyl ether into the reactor, and stir to make the sodium borohydride evenly disperse in the ethylene glycol dimethyl ether solution. Take by weighing 6.8g (44mmol) 3,5-dihydroxybenzoic acid is dissolved in the 34g ethylene glycol dimethyl ether solution, it is slowly added dropwise in the ethylene glycol dimethyl ether solution of sodium borohydride (within one hour After dripping), keep the temperature between 25-30°C. After the dropwise addition was complete, stirring was continued at room temperature for 1.5 hours. Next, 25 g (172 mmol) of boron trifluoride in ether was slowly added dropwise to the reaction system, and the addition was completed in about an hour, and the temperature was maintained at 25-30°C. After the dropwise addition was completed, the reaction was carried ...
Embodiment 2
[0036] Add 5g (35.7mmol) of 3,5-dihydroxybenzyl alcohol and 300mL of anhydrous acetonitrile successively into a 1L three-necked flask equipped with a thermometer and a condenser, start stirring, and add 12.2g (35.7mmol) of triphenyl Phosphine hydrobromide. The oil bath was heated to reflux, and the heating was stopped after 12 hours, and the reaction system was cooled to room temperature. Then the temperature in the reaction system was lowered to 0° C. under an ice-water bath, and continued to stir at this temperature for 30 minutes. At this time, it was found that a large amount of white solids were precipitated, filtered, and the filter cake was once cooled with low-temperature acetonitrile, and then Washed with anhydrous ether, and then dried, the dried solid product was 18.6g, and the yield was 72.3%.
[0037] 1 HNMR (300MHz, d 6-DMSO): d: 9.35ppm (2H, s), 7.94-7.63ppm (15H, m), 6.17-6.16ppm (1H, d, J = 2.09Hz), 5.82-5.80ppm (2H, t, J = 2.24Hz),4.92-4.87(2H,d,J=15.64Hz...
Embodiment 3
[0039] The solid 9.31g (20mmol) obtained in Example 2, 2.44g (20mmol) of p-hydroxybenzaldehyde, 7.41g (66mmol) of potassium tert-butoxide and 100 ml of dried anhydrous tetrahydrofuran were added to the reactor, while Heat up to 80°C while stirring, continue the reaction at this temperature until the reaction is substantially complete (about 20-24 hours), cool to room temperature, add 6g (5.2ml, 60mmol) of 36.5% concentrated hydrochloric acid, and continue stirring at room temperature React for 30 minutes. Filtrate, wash the filter residue with methyl tert-butyl ether, wash the filtrate with water, concentrate under reduced pressure to remove the solvent, and recrystallize the obtained crude product with ethanol-water to obtain a white solid of resveratrol, weight 3.42g, yield 75% .
[0040] 1 HNMR (300MHz, d 6 -DMSO)d9.59(bs,1H),9.23(bs,2H),7.42(d,J=8.70Hz,2H),6.97(d,J=16.5Hz,1H),6.85(d,J=16.5 Hz, 1H), 6.78(d, J=8.40Hz, 2H), 6.43(d, J=2.10Hz, 2H), 6.16(t, J=2.10Hz, 1H); 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com