Rhodamine B-based multifunctional fluorescent probe for recognizing Fe<3+>, Al<3+> and Cr<3+> ions and preparation method and application of rhodamine B-based multifunctional fluorescent probe for recognizing Fe<3+>, Al<3+> and Cr<3+> ions
A fluorescent probe, multi-functional technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of increasing detection costs, and achieve the effect of saving detection costs and saving detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthetic compound 2 The reaction formula is as follows:
[0052]
[0053] (1) Synthesis of compound 1
[0054] Put (3.0g, 6.8mmol) Rhodamine B in a round bottom flask, add 100mL of absolute ethanol to make it fully dissolved, then add 7.5mL of hydrazine hydrate, reflux and stir for 4h, after the reaction is over, the solvent ethanol is distilled off under reduced pressure. The remaining solid was acidified with an excess of 1mol / L HCl, and 0.5mol / L NaOH was adjusted to pH 7. At this time, a large amount of precipitate was precipitated, filtered with suction, washed the filter cake with deionized water, and dried in vacuum to obtain a yellowish solid compound 1.
[0055] (2) Synthesis of compound 2
[0056] Compound 1 (0.5g, 1mmol) was placed in a 100mL flask, and 30mL of dichloromethane was added to make it completely dissolved. Then 0.1 mL of chloroacetyl chloride was dissolved in a small amount of dichloromethane (5 mL). Under an ice bath, the mixed solution of chloroac...
Embodiment 2
[0058] Synthesize fluorescent probe L1, the reaction formula is as follows:
[0059]
[0060] The specific steps are:
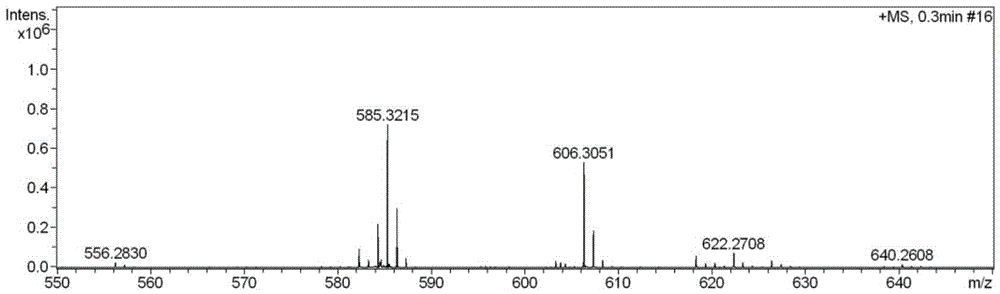
[0061] Take compound 2 (0.15g, 28mmol) dissolved in 20mL of acetonitrile, add morpholine (0.031g, 0.35mmol), KI (0.03g, 0.18mmol) and diisopropylethylamine (0.5mL, 2.9mmol), under nitrogen protection The reaction was stirred and refluxed at 70°C for 10 hours; after the reaction was completed, it was cooled to room temperature, the solvent was distilled off under reduced pressure, and column chromatography was used to separate the white solid fluorescent probe L1 with a yield of 85%.
[0062] Basic data of this rhodamine multifunctional fluorescent probe:
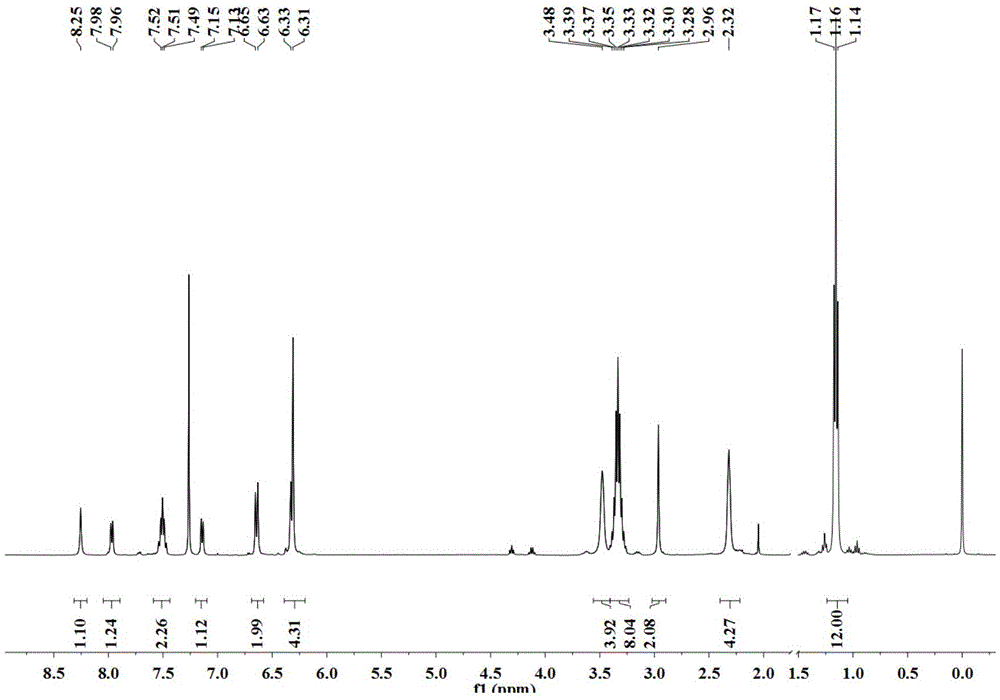
[0063] 1 HNMR(400MHz, CDCl 3 )δ8.25(s,1H),7.98(d,J=8.0Hz,1H),7.51-7.49(m,2H),7.33(t,J=7.5Hz,1H),7.15(d,J=6.7 Hz, 1H), 6.65 (d, J = 8.8 Hz, 2H), 6.32 (m, 4H), 3.48 (br, 4H), 4.53 (s, 2H), 3.38-3.28 (m, 8H), 2.96 (s ,2H),2.32(br,4H),1.17(t,J=7.0Hz,12H)(such as figure 1 ).
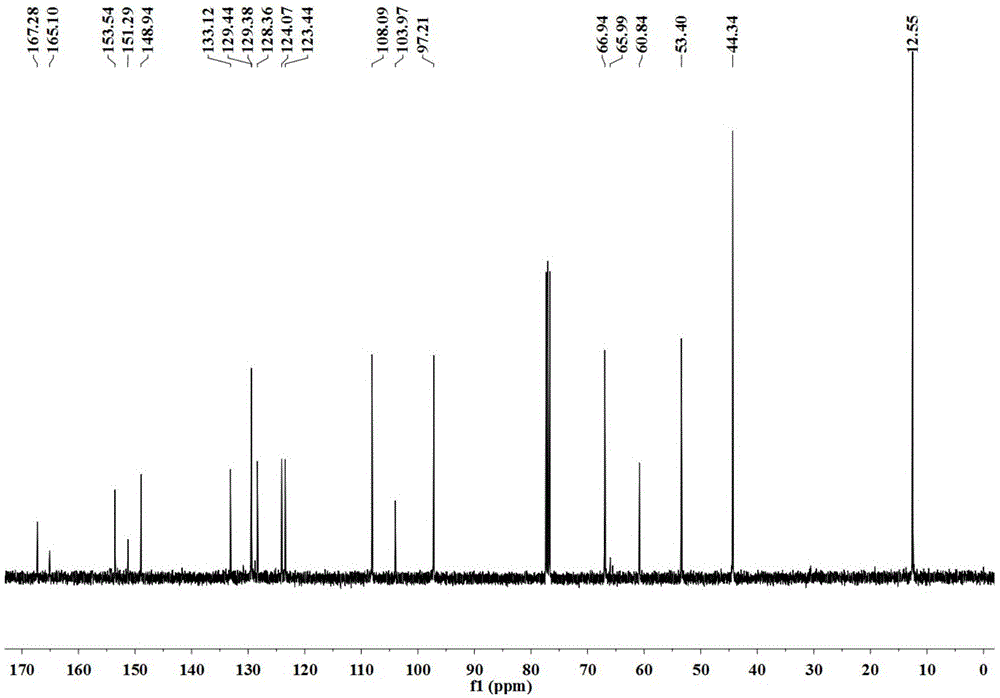
[0064] 13 CNMR(100.6MHz, CDCl 3 ) 167.3, ...
Embodiment 3
[0072] Example 3 Synthesis of Compound 3 And compound 6
[0073] The reaction formula is as follows:
[0074]
[0075] (1) Compound 3 Synthesis
[0076] Rhodamine B (444mg, 1.0mmol) and propargylamine (82.5mg, 1.5mmol), dissolved in dry dichloromethane, add benzotriazole-N,N,N',N'-tetramethylurea Hexafluorophosphate (HBTU) (568mg, 1.5mmol)) and triethylamine (152mg, 1.5mmol), react at room temperature for 15h, spin dry under reduced pressure, silica gel column chromatography to obtain a white solid 359mg, the yield was 75%.
[0077] (2) Compound 4 Synthesis
[0078] 2-Aminobenzothiazole (1g, 6.8mmol) and (0.65g, 8.2mmol) pyridine were dissolved in dry dichloromethane. Under ice bath conditions, bromoacetyl bromide (1.7g, 8.2mmol) was added dropwise. Methyl chloride solution. TLC tracking, silica gel column chromatography to obtain yellow solid compound 4 The yield was 85%.
[0079] (3) Compound 6 Synthesis
[0080] Compound 4 (270 mg, 1 mmol) and sodium azide (650 mg, 10 mmol) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com