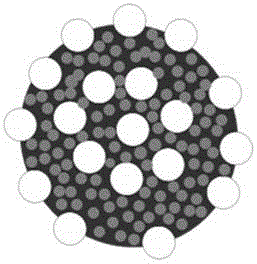
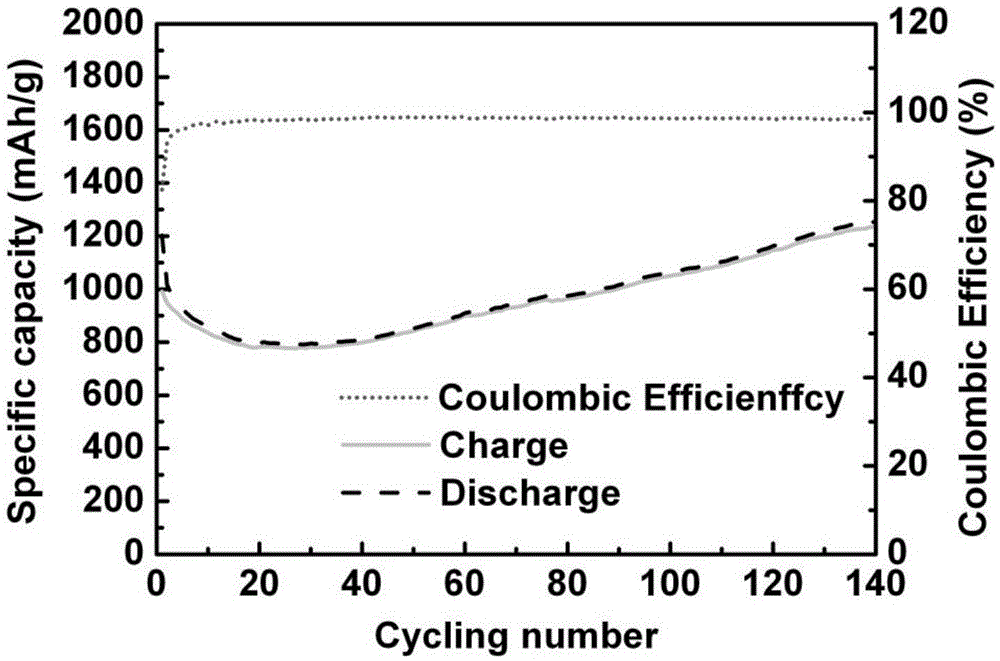
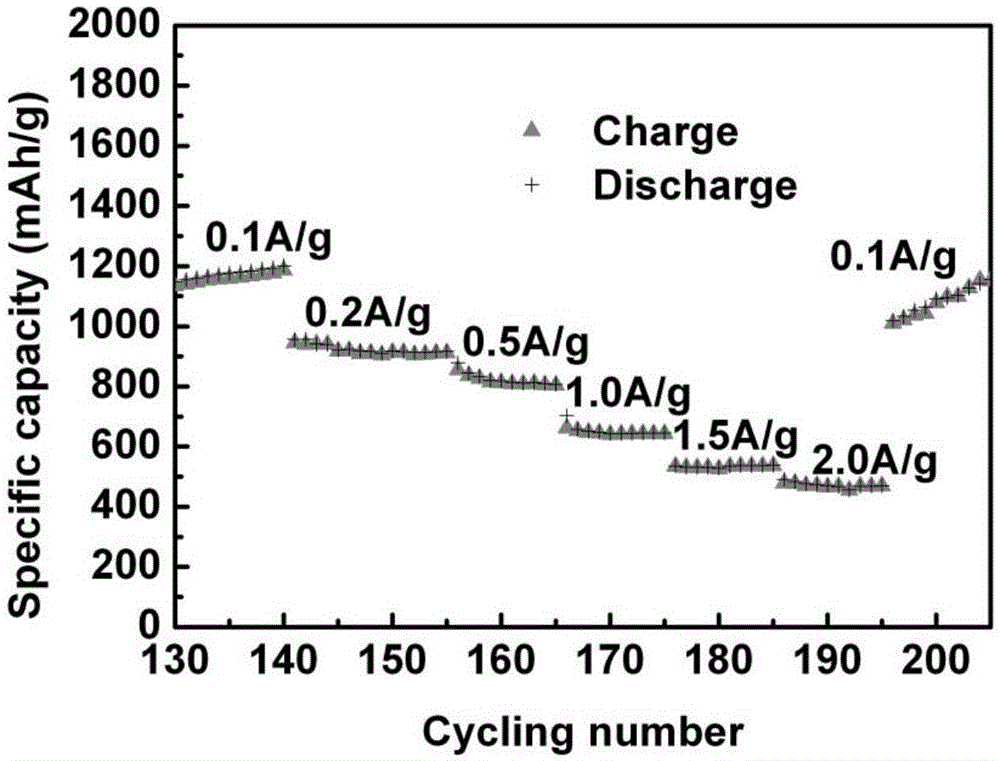
Carbon-coated porous manganese monoxide composite material and preparation method and application thereof
A manganese monoxide and composite material technology, applied in electrochemical generators, structural parts, electrical components, etc., can solve the problems of further increase in capacity and rapid loss, achieve good cycle stability, inhibit volume damage, prevent growing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A novel carbon-coated porous manganese monoxide core-shell composite material and its preparation method
[0034] 1. Dissolve 10mL styrene in 200mL ethanol, keep the 2After stirring for 30 min, the temperature was raised to 70°C and stabilized for 1 h to obtain solution Ⅰ.
[0035] 2. Take 0.273g of potassium persulfate into a 20mL volumetric flask, and slowly drop the potassium persulfate solution into solution Ⅰ. 2 The reaction was stirred at 70° C. for 12 h, cooled to room temperature, washed, and the solid reaction product was collected to obtain product I.
[0036] 3. Take 2g of product I, disperse it in 40mL of concentrated sulfuric acid by ultrasonic dispersion, and stir and react at 40°C for 3 hours. After the reaction is completed, add it to 100mL of water, let it stand and separate and wash , the solid product was collected to give product II.
[0037] 4. Disperse 0.5g of product II in 250mL of water, ultrasonically disperse for 30min, then slowly add 25mL ...
Embodiment 2
[0049] 1. Dissolve 20mL styrene in 300mL ethanol, keep the N 2 After stirring for 30 min, the temperature was raised to 70°C and stabilized for 2 h to obtain solution Ⅰ.
[0050] 2. Take 0.546g of potassium persulfate into a 20mL volumetric flask, and slowly drop the potassium persulfate solution into solution Ⅰ. 2 The reaction was stirred at 70° C. for 18 h, cooled to room temperature, washed, and the solid reaction product was collected to obtain product I.
[0051] 3. Take 3g of product I, disperse it in 75mL of concentrated sulfuric acid by ultrasonic dispersion, and stir and react at 40°C for 2 hours. After the reaction is completed, add it to 150mL of water, let it stand and separate and wash , the solid product was collected to give product II.
[0052] 4. Disperse 1g of product II in 280mL of water, ultrasonically disperse for 60min, then slowly add 35mL of ethanol, after complete dispersion, slowly add 230mL of 0.04MMnSO 4 solution and 0.4MNH 4 CO 3 solution, kep...
Embodiment 3
[0058] 1. Dissolve 20mL styrene in 400mL ethanol, keep stirring with helium for 60min, then raise the temperature to 70°C and stabilize for 1.5h to obtain solution Ⅰ.
[0059] 2. Take 0.546g of potassium persulfate into a 20mL volumetric flask, slowly drop the potassium persulfate solution into Solution I, stir and react for 24h in a helium atmosphere at 70°C, cool to room temperature, wash, and collect the solid reaction product. Product I is obtained.
[0060] 3. Take 4g of product I, disperse it in 120mL of concentrated sulfuric acid by ultrasonic dispersion, and stir and react at 40°C for 4 hours. After the reaction is completed, add it to 300mL of water, let it stand for separation and wash , the solid product was collected to give product II.
[0061] 4. Disperse 2g of product II in 300mL of water, ultrasonically disperse for 45min, then slowly add 50mL of ethanol, after complete dispersion, slowly add 250mL of 0.06MMnSO 4 solution and 0.6MNH 4 CO 3 Solution, keep st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Large hole diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com