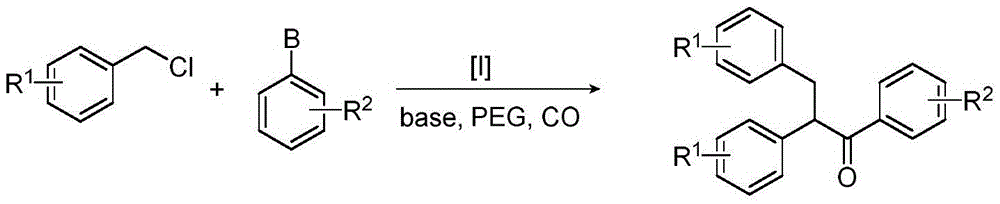
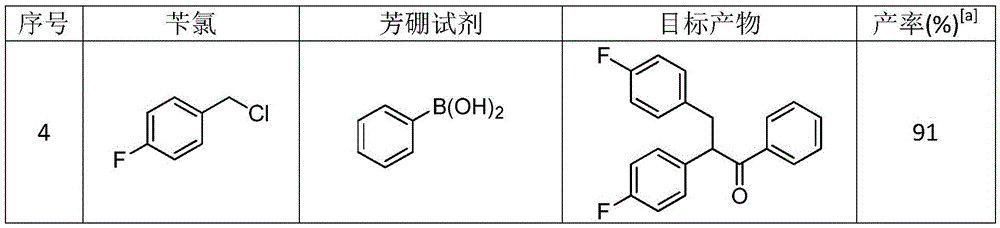
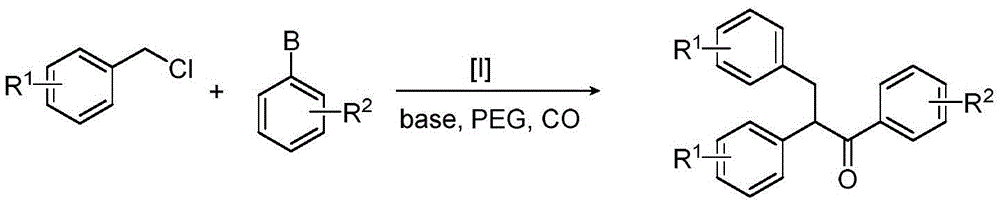
Method for synthesizing 1,2,3-triaryl-1-acetone compound from benzyl chloride through non-metal-catalyzed carbonylation
A carbonyl benzyl chloride, metal-free catalysis technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., can solve high requirements for reaction equipment, hinder wide application, and environmental problems and other problems, to achieve the effect of wide application range, good functional group compatibility and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Step 1: Connect the reaction bottle equipped with a stirring bar with high-purity carbon monoxide, and repeat three times of vacuumizing and filling with carbon monoxide, so that the reaction system is under a carbon monoxide atmosphere.
[0024] Step 2 Add sodium iodide (0.075mmol), sodium phosphate (2.0mmol), dipotassium hydrogen phosphate (0.1mmol), benzyl chloride (1.0mmol or 1.5mmol), aromatic boronic acid (0.5mmol) and poly Ethylene Glycol-400 (2.0 g). The reaction mixture was reacted at 100°C, and the progress of the reaction was monitored by thin-layer chromatography or gas chromatography.
[0025] After the reaction in step 3, cool to room temperature, extract with an organic solvent, combine the organic phases, concentrate and separate by column chromatography to obtain a pure product.
Embodiment 2
[0027] Step 1: Connect the reaction bottle equipped with a stirring bar with high-purity carbon monoxide, and repeat three times of vacuumizing and filling with carbon monoxide, so that the reaction system is under a carbon monoxide atmosphere.
[0028] Step 2 Add tetrabutylammonium iodide (0.075mmol), sodium carbonate (2.0mmol), benzyl chloride (1.0mmol or 1.5mmol), potassium arylfluoroborate (0.5mmol) and polyethylene glycol- 400 (2.0g). The reaction mixture was reacted at 100°C, and the progress of the reaction was monitored by thin-layer chromatography or gas chromatography.
[0029] After the reaction in step 3, cool to room temperature, extract with an organic solvent, combine the organic phases, concentrate and separate by column chromatography to obtain a pure product.
Embodiment 3
[0031] Step 1: Connect the reaction bottle equipped with a stirring bar with high-purity carbon monoxide, and repeat three times of vacuumizing and filling with carbon monoxide, so that the reaction system is under a carbon monoxide atmosphere.
[0032] Step 2 Add sodium iodide (0.075mmol), sodium phosphate (2.0mmol), dipotassium hydrogen phosphate (0.1mmol), benzyl chloride (1.0mmol or 1.5mmol), aromatic boronic acid (0.5mmol) and poly Ethylene Glycol-1000 (2.0 g). The reaction mixture was reacted at 100°C, and the progress of the reaction was monitored by thin-layer chromatography or gas chromatography.
[0033] After the reaction in step 3, cool to room temperature, extract with an organic solvent, combine the organic phases, concentrate and separate by column chromatography to obtain a pure product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com