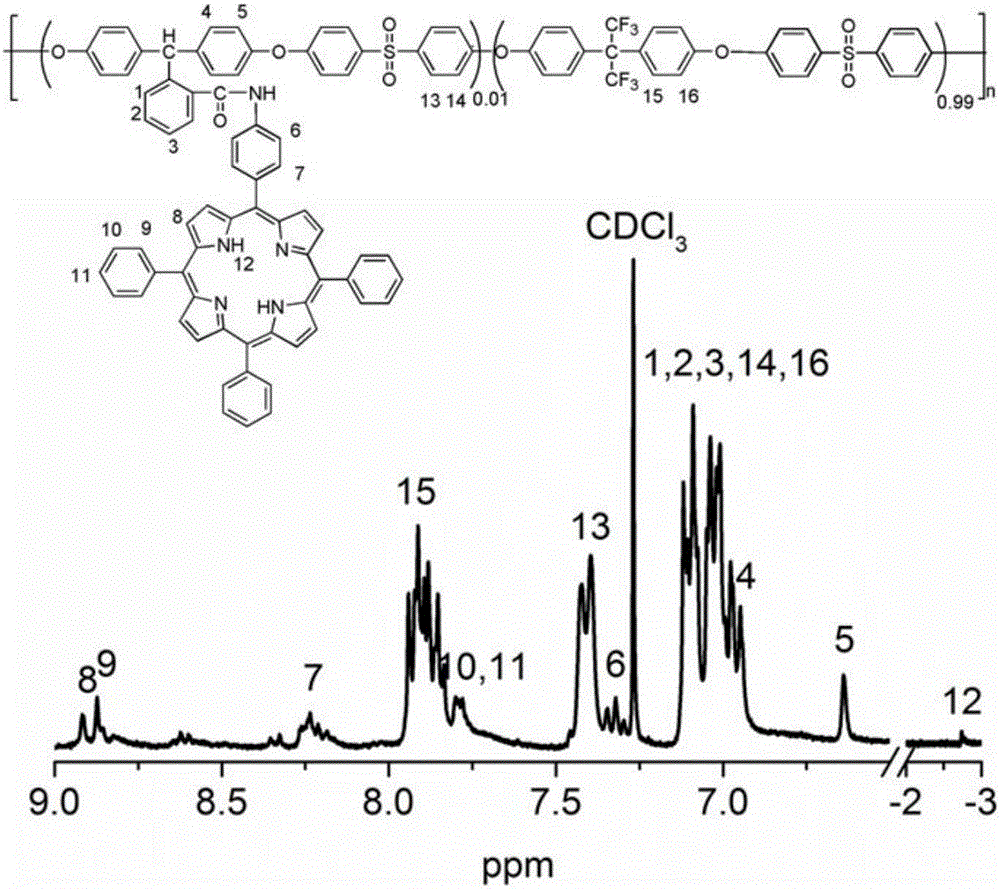
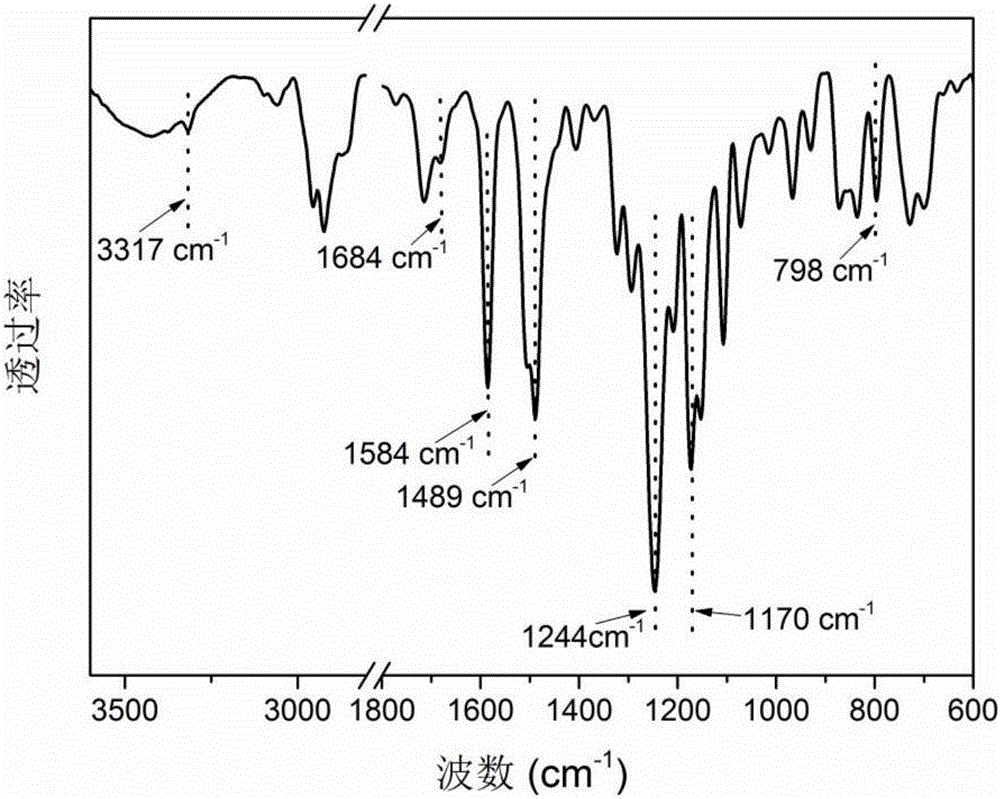
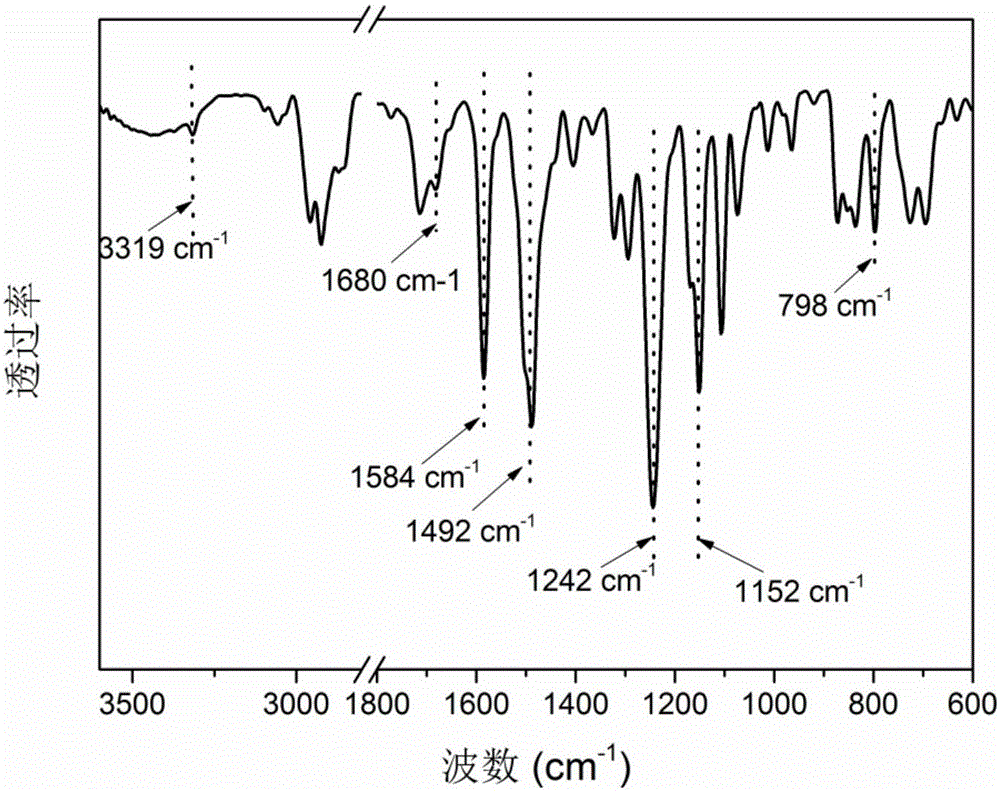
Polyether sulphone copolymer with side chains containing porphyrin and preparation method of polyether sulphone copolymer
A technology of polyaryl ether sulfone and copolymer, applied in the field of polyaryl ether sulfone copolymer and its preparation, can solve the problems of low melting point and hardness, easy stacking and aggregation, etc., and achieve the effect of excellent optical limiting performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation and purification of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin: In a 500mL three-neck round bottom flask, add 2g of 5,10,15,20-tetraphenyl Porphyrin, 200mL chloroform, electric stirring, ice-salt bath cooling to about 0°C, dissolving 1.10mL (27mmol) fuming nitric acid in 30mL chloroform, adding dropwise for 1.5h, continuing to react for 2h, using TLC to detect the reaction until four The phenylporphyrin completely disappeared and the reaction was stopped. Then use 0.5mol / L sodium hydroxide to neutralize to pH=8, wash with water (150mL×6) to collect the organic phase, and wash the organic phase with anhydrous MgSO 4 Dry for 8h and concentrate. Separation by silica gel column chromatography to obtain purple crystal 5-(4-nitrophenyl)-10,15,20-triphenylporphyrin 1.24g, then add 1g5-(4 -Nitrophenyl)-10,15,20-triphenylporphyrin, add 80mL of concentrated hydrochloric acid, pass nitrogen gas for 10min, stir electrically, add 2g of stannous chloride, heat in...
Embodiment 2
[0047] (1) The preparation of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin was as described in step (1) in Example 1.
[0048] (2) In a 100mL three-neck flask equipped with a mechanical stirrer, a nitrogen inlet, a thermometer, a water dispenser and a condenser tube, add 5.085g (20mmol) of 4,4'-difluorodiphenylsulfone, 2,2-bis (4-Hydroxyphenyl) hexafluoropropane 5.3797g (16mmol), phenolphthalein 1.2814g (4mmol) and anhydrous potassium carbonate 3.649g (26.4mmol) are dissolved in sulfolane (34mL), add water-carrying agent toluene (17mL), Nitrogen was introduced, and after all the monomers were dissolved, the temperature was raised to 120°C and refluxed for 3 hours, then the water and water-carrying agent toluene generated by the reaction were removed, and the temperature of the reaction system was continued to rise to 175°C, and the polymerization reaction was completed after 5 hours. Slowly pour the viscous solution into 5% hydrochloric acid aqueous solution by mass fraction ...
Embodiment 3
[0055] (1) The preparation of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin was as described in step (1) in Example 1.
[0056] (2) In a 100mL three-neck flask equipped with a mechanical stirrer, a nitrogen vent, a thermometer, a water tank and a condenser, add 5.085g (20mmol) of 4,4'-difluorodiphenylsulfone, 2,2-diphenylsulfone (4-Hydroxyphenyl)propane 2.740g (14mmol), phenolphthalein 2.563g (6mmol) and anhydrous potassium carbonate 3.18g (23mmol) were dissolved in sulfolane (30mL), water-carrying agent toluene (15mL) was added, and nitrogen was introduced After all the monomers are dissolved, heat up to 130°C and reflux for 3 hours, then remove the water and water-carrying agent toluene generated by the reaction, continue to raise the temperature of the reaction system to 175°C, and complete the polymerization reaction after 5 hours. The viscous solution was slowly poured into 5% hydrochloric acid aqueous solution to obtain flexible polymer strands, which were pulverized int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com