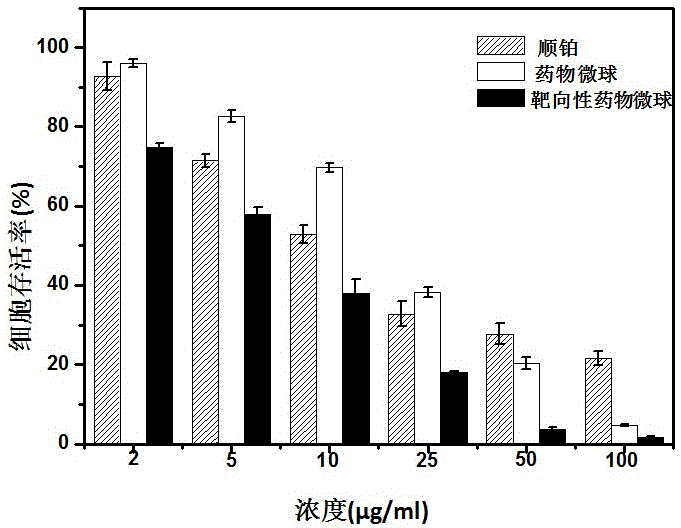
Anti-tumor platinum pro-drug and nanometer hydrogel drug and preparation method thereof
A prodrug and platinum-based drug technology, applied in the field of biomedicine, can solve the problems of fast metabolism, short cycle time, poor targeting, etc., achieve clear and concise preparation process, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of cisplatin antitumor prodrugs
[0031]Add 2g of cisplatin, 50ml of deionized water and 11.4ml of hydrogen peroxide (30%) into a 100ml three-neck round bottom flask, and react in a nitrogen atmosphere at 70°C in the dark for 6h, then cool down to room temperature for another 12h, and spin evaporate After part of the water, the product was cooled with ice water to precipitate the product, suction filtered, and the reaction product was washed with ice water, ethanol, and ether once and then vacuum-dried to obtain a Pt(IV) precursor modified with hydroxyl groups at both ends of the axial direction, which was recorded as Pt(IV)-( Oh) 2 -1.
[0032] Take the above 2g precursor Pt(IV)-(OH) 2 -1 into a 50ml three-neck round bottom flask, then add methacrylic anhydride and 10ml pre-dried dimethyl sulfoxide (DMSO) at a molar ratio of 1:2, and react for 24 hours under a nitrogen atmosphere and 60°C in the dark After the reaction, the solvent was removed...
Embodiment 2
[0033] Example 2: Preparation of Oxaliplatin Antitumor Prodrugs
[0034] Add 2 g of oxaliplatin, 50 ml of deionized water, and 11.4 ml of hydrogen peroxide (30%) into a 100 ml three-neck round bottom flask. Under a nitrogen atmosphere, react at 70 ° C for 6 h in the dark, then cool down to room temperature for another 12 h, spin After evaporating part of the water, cool and precipitate the product with ice water, filter it with suction, wash the reaction product once with ice water, ethanol, and ether, and then dry it in vacuum to obtain a Pt(IV) precursor modified with hydroxyl groups at both ends of the axial direction, denoted as Pt(IV) -(OH) 2 -2
[0035] Take the above 2g precursor Pt(IV)-(OH) 2 -1 into a 50ml three-neck round bottom flask, then add methacrylic anhydride and 10ml pre-dried dimethyl sulfoxide (DMSO) at a molar ratio of 1:2, and react for 24 hours under a nitrogen atmosphere and 60°C in the dark After the reaction, the solvent was removed by lyophilizati...
Embodiment 3
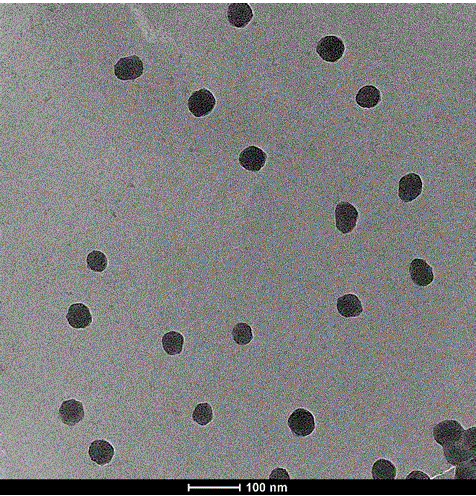
[0036] Example 3: Preparation of Oxaliplatin Antitumor Prodrug Nano Hydrogel Microspheres
[0037] MAA monomer 400mg, ZDMA cross-linking agent 100mg, AIBN initiator 16.7mg, acetonitrile 40mL, heated to 95°C, reflux solvent reaction for 2 hours, centrifuged to remove solvent and unreacted monomer, washed 3 times with ethanol and deionized water, Dry in a vacuum oven for 24 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com