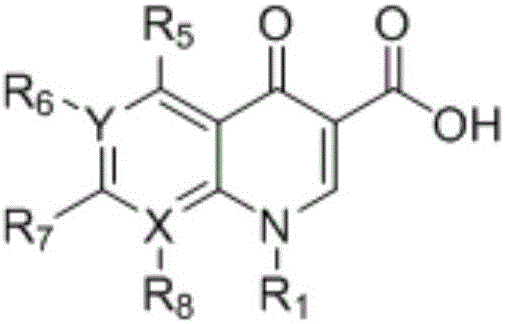
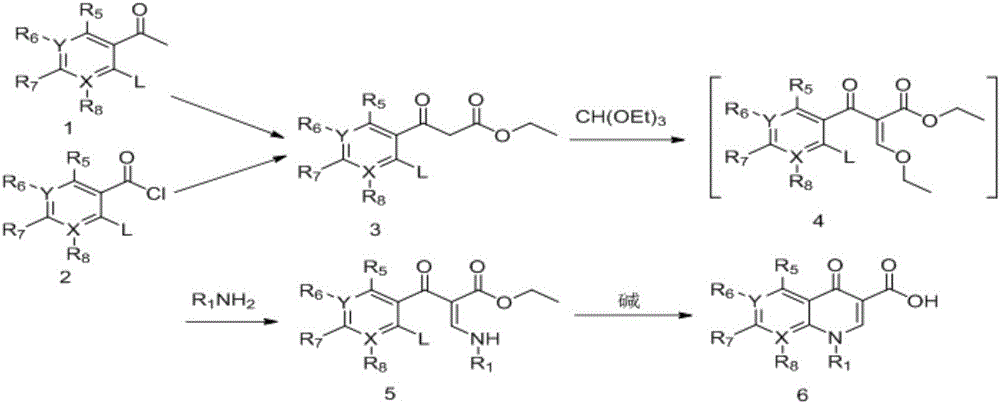
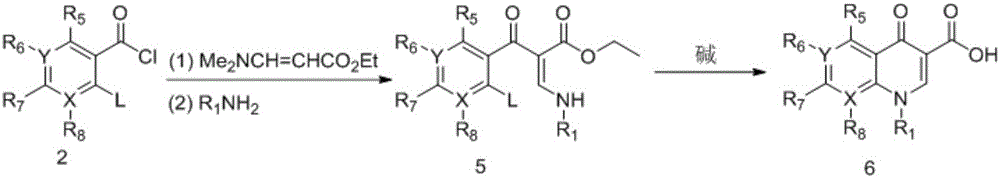
Synthesizing method of 3-N,N-dimethylamino ethyl acrylate
A technology of ethyl dimethylamino acrylate and a synthesis method, applied in the field of 3-N, can solve the problems of unsatisfactory product purity and yield, influence of high-purity product yield, incomplete reaction, etc., and achieves improvement of raw material conversion rate , the effect of easy large-scale processing and manufacturing, reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] S1, intermediate preparation reaction step: add 300mL toluene and 14g metallic sodium into a 500mL reaction bottle, and heat to melt. Stirring was started, and 40 g of ethanol was added dropwise. After the dropwise addition was completed, reflux was stirred for 1 hour, and the temperature was lowered to obtain a suspension of sodium ethoxide in toluene.
[0050] S2, condensation reaction step: transfer the above-mentioned sodium ethoxide toluene suspension into a 1L autoclave, add 80g ethyl acetate and 1.5g Raney nickel, close the autoclave, and start stirring; the autoclave is replaced with carbon monoxide gas after nitrogen replacement, and The pressure rises to 3-5MPa, heats to 60-70°C, stirs for 6 hours, cools down and releases the pressure.
[0051] S3, amine substitution reaction step: add 210 g of 35% dimethylamine hydrochloride aqueous solution into a 1L reaction bottle, start stirring, add the condensation reaction solution in batches at room temperature, conti...
Embodiment 2
[0054] S1, intermediate preparation step: directly add 75 g of commercially available potassium tert-butoxide solid to the toluene suspension to form an intermediate.
[0055] S2, condensation reaction step: move the above-mentioned intermediate into a 1L autoclave, add 60g of ethyl acetate and 2g of cobalt carbonyl, put together the kettle, and start stirring; the autoclave is replaced with nitrogen and then replaced with carbon monoxide gas, and the pressure rises to 1.5-2.5 MPa, heat to 80-90°C, stir for 6 hours, cool down and release the pressure.
[0056] S3, amine substitution reaction step: Add 170 g of 45% dimethylamine hydrochloride aqueous solution into a 1L reaction bottle, start stirring, add the condensation reaction solution in batches at room temperature, and continue stirring for 2 hours after the addition is complete. Then stop stirring, filter with suction, separate the layers of the filtrate, concentrate the organic phase, and rectify to obtain 3-N,N-ethyl d...
Embodiment 3
[0058] S1, intermediate preparation steps: add 200mL ethanol to a 500mL reaction bottle, add sodium metal in batches, continue stirring until the sodium metal is completely reacted after the addition, and lower the temperature to obtain a sodium ethoxide ethanol suspension.
[0059] S2, condensation reaction step: transfer the above-mentioned sodium ethoxide ethanol suspension into a 1L autoclave, add 60g of ethyl acetate and 1g of rhodium chloride, combine the kettle, and start stirring; the autoclave is replaced with carbon monoxide gas after nitrogen replacement, and pressure Rise to 4-5MPa, heat to 70-80°C, stir for 4 hours (cool down, release pressure.
[0060] S3, amine substitution reaction step: add 300 g of 20% dimethylamine hydrochloride aqueous solution into a 1L reaction bottle, start stirring, add the condensation reaction solution in batches at room temperature, continue stirring for 2 hours after the addition, and then stop stirring, After suction filtration, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com