LC-MS/MS combined method for determining impurity content in lapatinib
A technology of lapatinib and fluorobenzyloxy, applied in the field of drug analysis, can solve the problems such as no method for the determination of the content of compound 4 and compound 9 that has been reported, and the impurity content analysis and determination method needs to be improved, and the detection result is achieved. Accurate and reliable, easy to operate, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
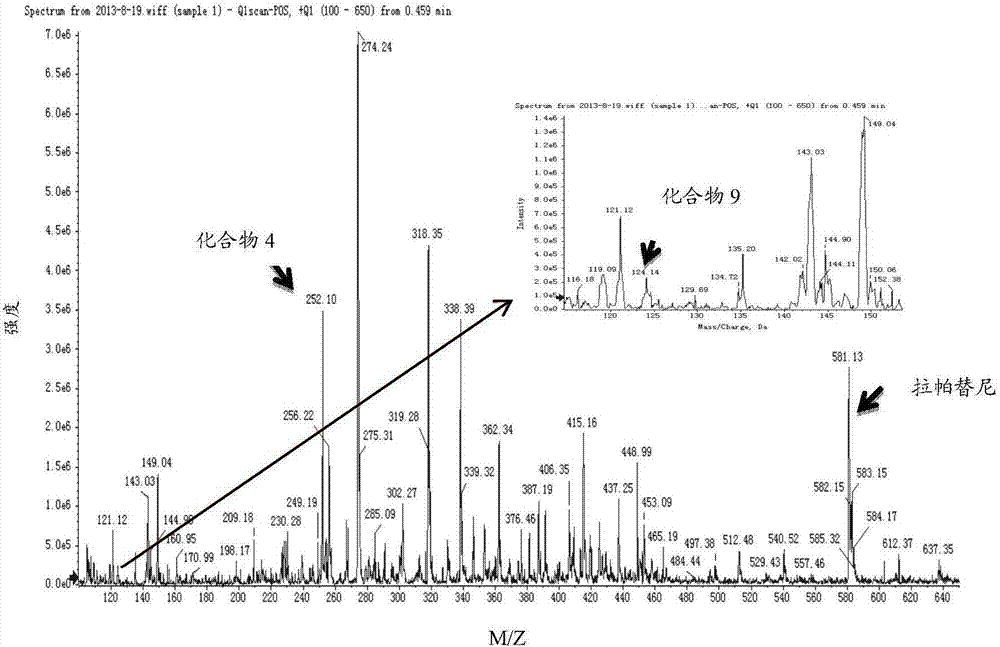
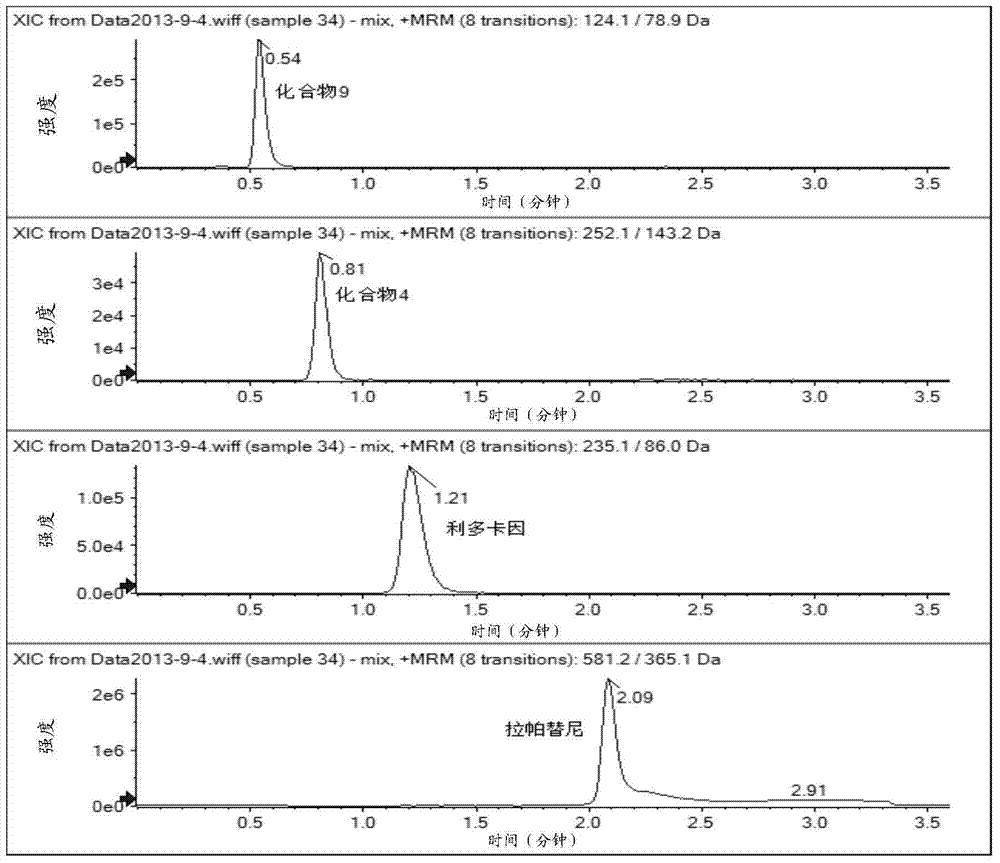
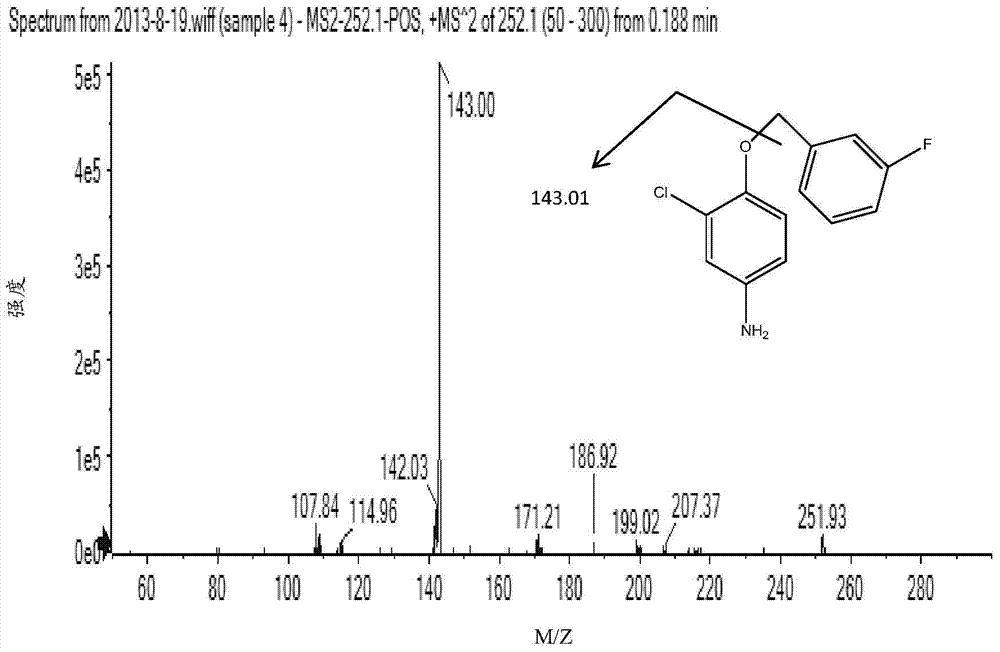
[0066] Use the mass spectrometer (AB Sciex Q-Trap 4500) to perform a full scan (Q1Scan) on compound 4, compound 9 and lapatinib in positive ion mode, find the corresponding parent ion, and then do the corresponding secondary ion on the parent ion , select the product ion with higher intensity as the final detection ion, the spectrum is shown in Figure 2 to Figure 5 . Finally, each compound selects an ion pair with higher intensity, and optimizes the corresponding parameters (ion source parameters, parameters related to the compound). The results are shown in Table 3 and Table 4.
[0067] Table 3: Ion source parameters
[0068] polarity
Positive pole
scan type
MRM
Curtain gas (CUR)
30psi
Ion source (GS1)
50psi
Ion source (GS2)
60psi
ion ejection voltage
5500V
temperature
550℃
[0069] Table 4: Compound-related parameters
[0070]
[0071]
Embodiment 2
[0073] Using 5 types of chromatographic columns (Synergi Hydro-RP, 50*3.0, 4μm; Synergi Polar-RP 50*3.0, 4μm; Aquasil C1850*2.1mm, 5μm; Kromasil silica 50*3.0mm, 5μm; Betasil silica-10050 *2.1mm, 5μm), with acetonitrile as mobile phase B and water (containing 5mM ammonium formate) as mobile phase A, compound 4 and compound 9 were detected by LC-MS / MS respectively, and the chromatograms were recorded.
[0074] result:
[0075] When Hydro-RP and Polar-RP columns were used, compound 9 was basically not retained on Hydro-RP and Polar-RP columns.
[0076] When using Aquasil C18 chromatographic column, compound 9 was not retained when low organic phase (20% acetonitrile isocratic elution) was used, but compound 9 was retained in high organic phase (95% acetonitrile isocratic elution).
[0077] When using Betasil silica-100 column and Kromasil silica column, the retention of compound 9 was strong, but the retention of compound 4 was not.
[0078] Therefore, the present invention pr...
Embodiment 3
[0079] Embodiment 3: methodological investigation and sample analysis
[0080] According to the limit of compound 4 is not higher than 4ug / 1g, the limit of compound 9 is not higher than 0.02%, the calibration range of the two compounds is respectively 0.200ng / ml-10.0ng / ml (compound 4), 10ng / ml ml—500ng / ml (compound 9). Instruments and reagents
[0081] Reagents: acetonitrile (Fisher), water, ammonium formate, lapatinib API (14 batches), 4-(3-fluorobenzyloxy)-3-chloroaniline (batch number: 120814), 2-( Thiamyl) ethylamine hydrochloride (batch number: 20120521CMQA-1-MZP-01-014).
[0082] Instrument: Mass spectrometer: AB Sciex Q-Trap 4500,
[0083] HPLC: Shimadzu 20ACXR
[0084] (1) Preparation of reference substance stock solution: Take an appropriate amount of reference substance (compound 4, compound 9) and dissolve it in a solvent (acetonitrile: water = 4:1) to prepare a 1.00 mg / ml stock solution. Then compound 4 was diluted with acetonitrile: water (1:1) to an intermed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com