Fluorescein diacetate fluorescence probe containing aldehyde group structure and preparation method and application thereof
A fluorescein diacetate, fluorescent probe technology, used in fluorescence/phosphorescence, biochemical equipment and methods, chemical instruments and methods, etc., can solve problems such as unsolved problems, leakage of fluorescent products, etc. Achieve good chemical stability, good solubility and biocompatibility, and enhance the effect of specific selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 A kind of preparation method of fluorescein diacetate fluorescent probe (A-FDA fluorescent probe) containing aldehyde structure
[0022] Add 1 mol of fluorescein monoaldehyde and 2 mol of potassium carbonate to 3 mol of ether, add 3 drops of dichloromethane until dissolved, add 5 ml of acetic anhydride dropwise, and react with magnetic stirring for 6 hours at room temperature, filter the reactant with suction, and remove the filtrate. Spin-dry, and purify by silica gel column chromatography to obtain a white solid, which is the target product A-FDA fluorescent probe.
[0023] HRMS: 444.0845.
[0024] The preparation reaction formula is as follows:
[0025]
[0026] Among them, the synthesis method of fluorescein monoaldehyde is as follows: take 2g of fluorescein in a flask, add 12ml of chloroform and 0.4ml of 15-crown-5 into the flask, slowly add NaOH solution dropwise to the flask, put it in an oil bath at 55°C, and react for 7 hours , take the produc...
Embodiment 2
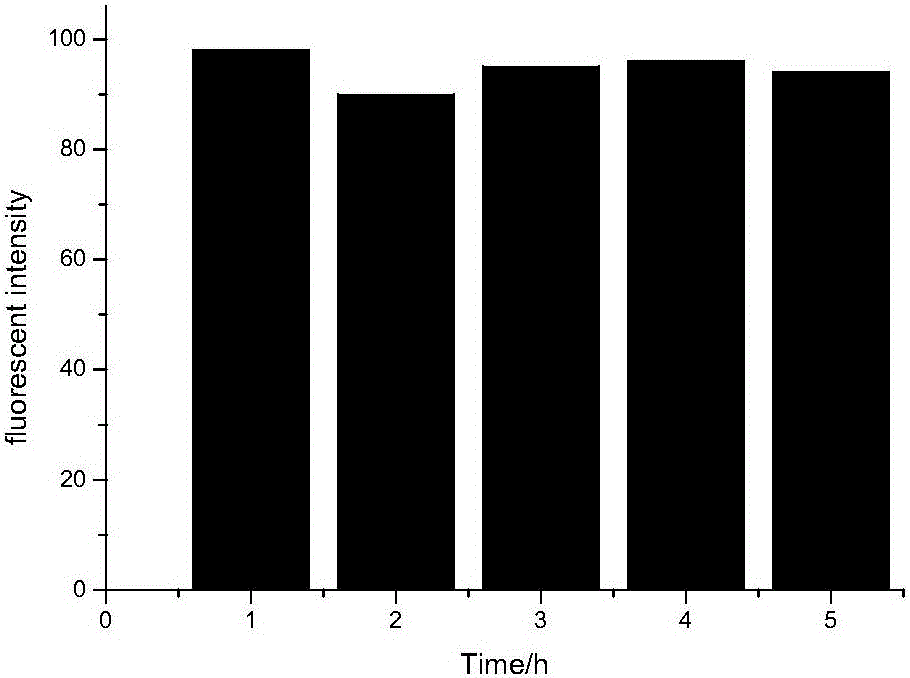
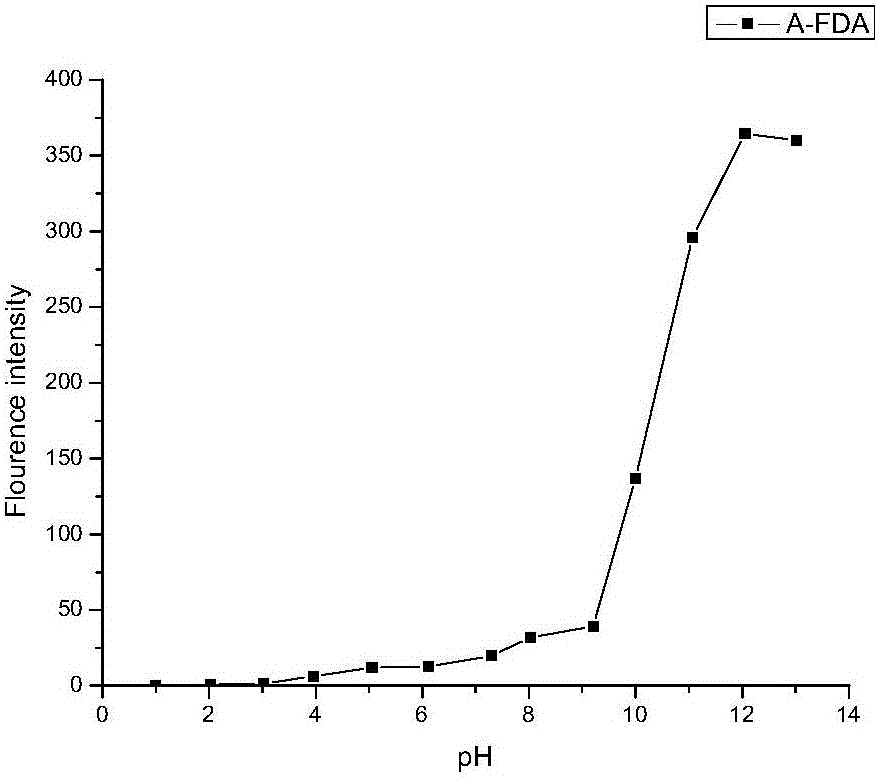
[0033] Embodiment 2, A-FDA fluorescent probe measures soil hydrolytic enzyme
[0034] Take 2g of soil in a 125ml Erlenmeyer flask, add 15ml of phosphate buffer solution (pH=7.4, 60mM) into the Erlenmeyer flask, add 0.2ml of A-FDA solution (3mM) into the Erlenmeyer flask, and cultivate at 30°C Add a terminator (a mixed solution of chloroform and methanol at a volume ratio of 2:1) in 1h, take the sample in a 15ml centrifuge bottle, rotate at 2000, centrifuge for 3min, filter the supernatant, and test, the result is as follows: Figure 4 Ac 1 ; set the amount of the control group, one group is: take 2g of soil in the Erlenmeyer flask, add 15ml of phosphate buffer solution (pH=7.4, 60mM), add 0.2ml of acetone into the Erlenmeyer flask, culture at 30°C for 1h with constant temperature shaking, add Terminator, centrifuged to get the supernatant, tested, the results are as follows Figure 4 Ab 1 The other group is: add the same amount of phosphate buffer solution and 0.2ml A-FDA s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com