Chlorotoxin polypeptide-ferritin heavy chain fusion protein, self-assembled protein nanocage and its preparation method and application
A technology of ferritin heavy chain and chlorotoxin, applied in the field of genetic engineering, can solve the problems of weakened biological activity, insufficient stability, poor pharmacokinetics and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1: molecular design CHF gene
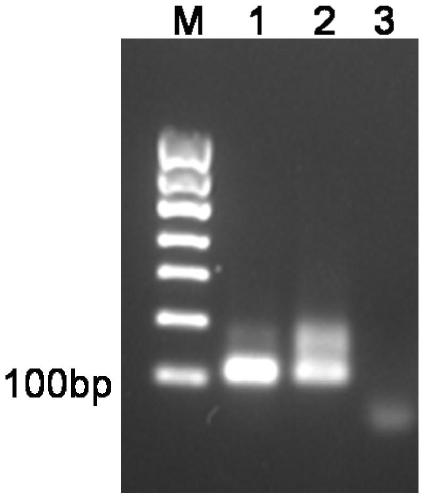
[0096] A. Design primers: Using the human iron protein nanocage heavy chain in the human genome cDNA library as a template, design 2 primers to amplify the HFt gene by PCR, and the forward primer is primer HFP1:TTA GGATCC ATGACCACCGCGAGCACCA (SEQUENCENO.13), the forward primer is primer HFP2: TTA CTCGAG GCTTTCGTTATCGCTATCGCCC (SEQUENCE NO. 14). In addition, two PCR amplification primers were designed for PCR amplification to obtain the CTX gene, and the forward primer was primer CP1: TTA CCATGG GCATGTGTATGCCGTGCTTCACTACCGATCA (SEQUENCE NO.15), the reverse primer is primer CP2: TTA GGATCC ACGGCACAGACACTGCGGACCGT (SEQUENCE NO. 16).
[0097] B. PCR amplification to obtain the HFt gene: 5 microliters of 10xTaq polymerase buffer, 4 microliters of dNTP mixture, 1 microliter of primer HFP1, 1 microliter of primer HFP2, and 1 microliter of template (cloning to obtain plasmid Template, the amino acid sequence is:
[0098]MTTASTS...
Embodiment 2
[0101] Embodiment 2: Construction of CHF protein nanocage recombinant expression vector
[0102] A: Double digestion and ligation of HFt, CTX gene and pET-28a vector
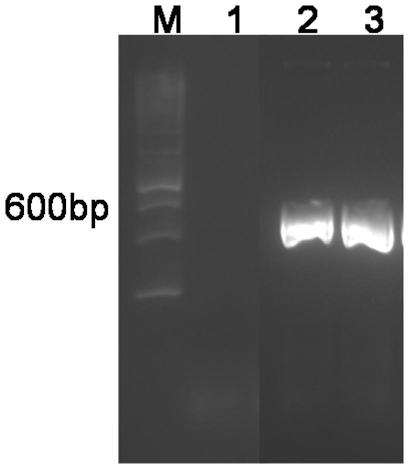
[0103] The PCR product obtained in Example 1 was extracted with phenol:chloroform:isoamyl alcohol (25:24:1), precipitated with absolute ethanol (2.5 volumes), and then dissolved with 50 μl of sterile water. The recovered PCR product and expression vector pET-28a plasmid were digested with restriction endonucleases BamHI and HindIII (products of Takara Company). Digestion reaction: 1 μl each of BamHI (14U / μl) and HindIII (20U / μl), 2.5 μl of 10-fold buffer, 50-100 ng of PCR product or pET-28a plasmid, and sterile water to a total volume of 25 μl. 37 ℃ water bath for 5 hours, the digested product was extracted with phenol: chloroform: isoamyl alcohol, precipitated with absolute ethanol (2.5 times volume) and washed with T 4 DNA ligase (product of Takara Company) ligated the PCR product to the expression vector pE...
Embodiment 3
[0110] Embodiment 3: the preparation of recombinant CHF genetically engineered bacteria
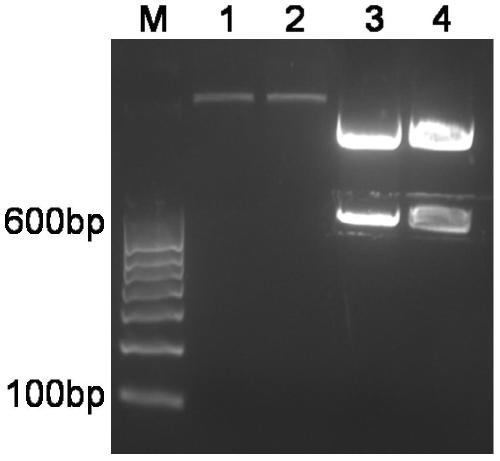
[0111] The positive clones sequenced correctly in step 2C of Example 2 were extracted by the alkaline lysis method to extract the recombinant expression plasmid pET-28a-CHF (see the second edition of "Molecular Cloning" for the method). The extracted pET-28a-CHF plasmid was transformed into Escherichia coli competent BL21 (DE3) cells prepared in the step of Example 2B according to the transformation method in the step of Example 2C. Tablet is LB / Kan + Agar plates. Single clones were picked to obtain genetically engineered bacteria BL21(DE3)(pET-28a-CHF).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com