Preparation method, product and application of single-modified polyethylene glycol recombinant human erythropoietin
A technology of polyethylene glycol and erythropoietin, which is applied to the preparation method of peptides, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of high multiple substitution rate, difficult purification, single substitution Low efficiency and other problems, to achieve the effect of simple process, conducive to purification, high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Preliminary Example 1 Recombinant Human Erythropoietin (EPO) Stock Solution
[0037] EPO stock solution is made by using recombinant DNA technology to transfect the recombinant plasmid with human erythropoietin gene into CHO-dhfr- (dihydrofolate reductase gene-deficient cells) cell line, and make it after cell culture, separation and high purification. details as follows:
[0038] 1. Basic requirements of raw materials and auxiliary materials
[0039] Production and testing facilities, raw materials and auxiliary materials, water, utensils, animals, etc. should meet the relevant requirements of the current version of the "Chinese Pharmacopoeia" three "general rules".
[0040] 2. Preparation of EPO stock solution
[0041] 2.1 Engineered cells
[0042] 2.1.1 Name and source
[0043] The polyethylene glycol recombinant human erythropoietin engineering cell line is a CHO-dhfr- (dihydrofolate reductase gene-deficient cell) cell line transfected with a reco...
Embodiment 2
[0085] Example 2 Identification of single-modified polyethylene glycol recombinant human erythropoietin—PEG modification reaction
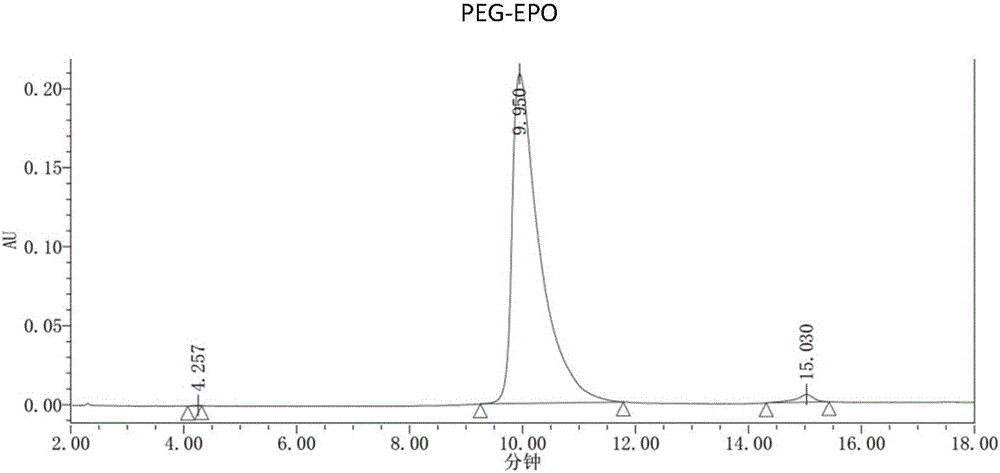
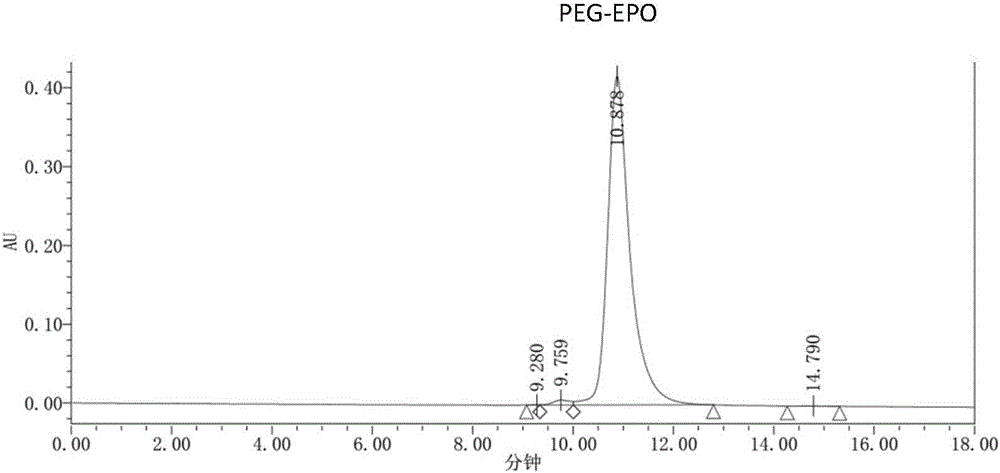
[0086] The PEG modification ratio of the single-modified polyethylene glycol recombinant human erythropoietin prepared in Example 1 was identified by reversed-phase-high performance liquid chromatography. Mobile phase A is trifluoroacetic acid aqueous solution (0.3% TFA); Mobile phase B is trifluoroacetic acid and acetonitrile aqueous solution (0.2% TFA, 84% acetonitrile); Flow rate is 1ml / min; Detection wavelength is 220nm; Column temperature is 60 ℃ ; The injection volume is 5-20μg. The chromatographic column adopts Agilent Poroshell300SBC8 (5μm, 300A, 2.1x75mm).
[0087] Elution gradient:
[0088]
[0089]
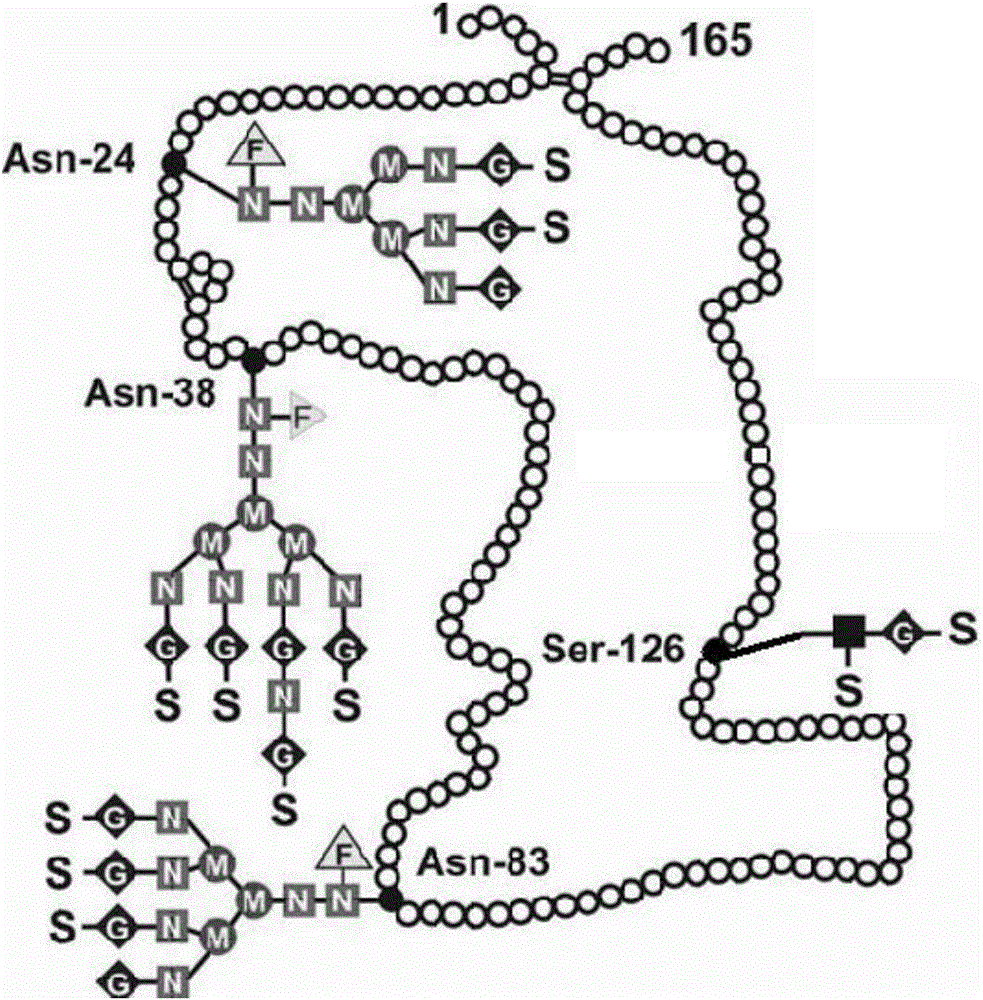
[0090] The identification results of PEG modification ratio are shown in image 3 And table 1, target product single modification PEG-EPO (structural formula sees Figure 4 ) was 42.91%.
[0091] Chromatographic peak results of t...
Embodiment 3
[0093] Example 3 Identification of Single Modified Polyethylene Glycol Recombinant Human Erythropoin—Purification of PEG-EPO
[0094] The monomodified polyethylene glycol recombinant human erythropoietin prepared in Example 1 was purified. Assemble an ion-exchange chromatography column, choose strong cationic medium MacroCapSP as filler, the column height is about 15cm, connect an online ultraviolet detector behind the column, and carry out chromatographic purification in an environment of 2-8°C.
[0095] Equilibrate not less than 5 column volumes with 30 mM NaAc (sodium acetate), pH 4.0 buffer. Load the modified reaction solution prepared in Example 1, and the loading amount should not exceed 2 mg protein / ml medium (optimally about 1 mg protein / ml medium). Equilibrate no less than 5 column volumes with 30mM NaAc, pH 4.0 buffer after loading.
[0096] Wash 3-4 column volumes with 100mM NaCl, 30mMNaAc, pH4.0; elute with 175mMNaCl, 30mMNaAc, pH4.0 for 3 column volumes, collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com