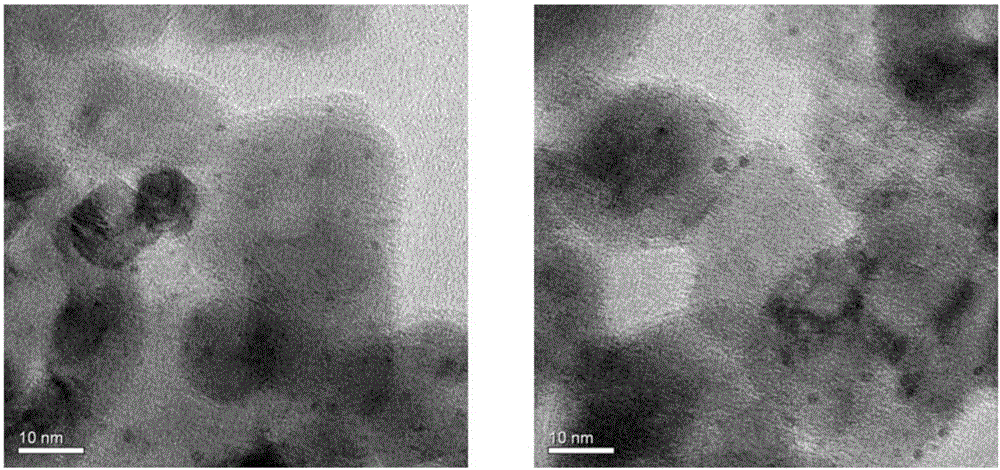
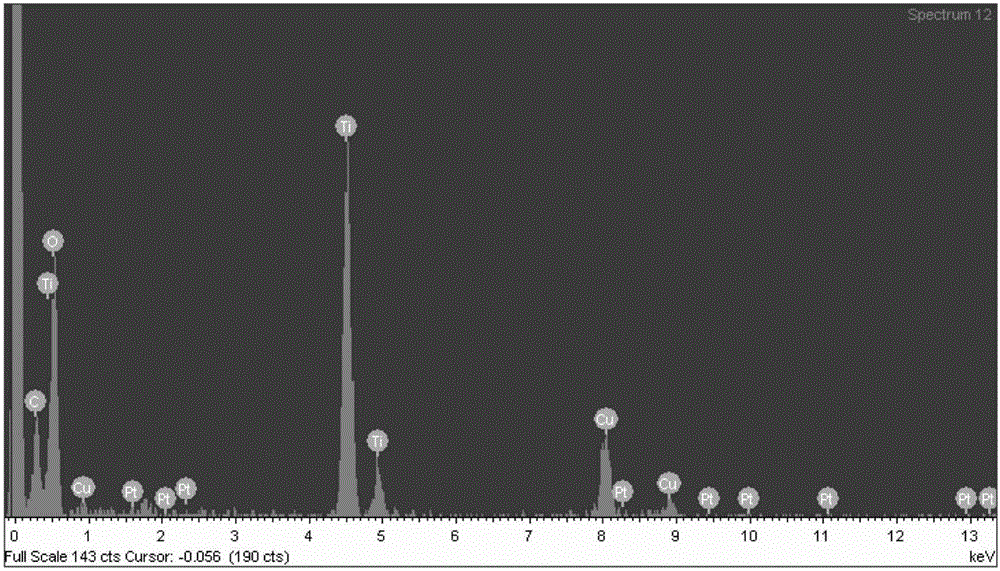
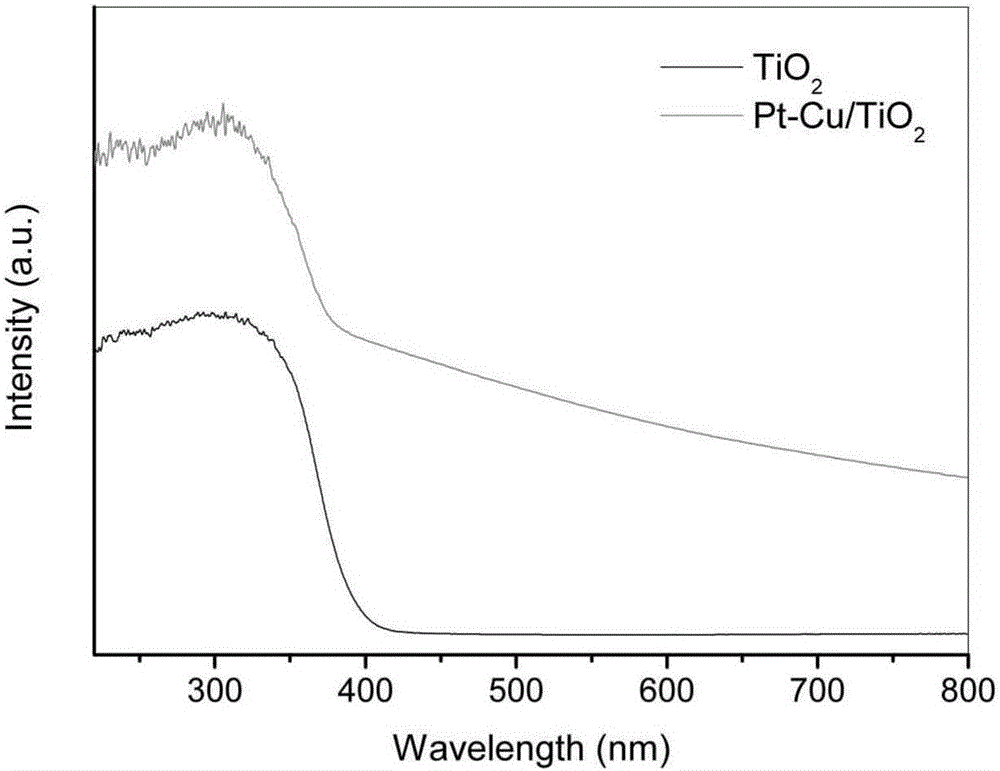
Method for preparing benzimidazole compound through supported bimetallic catalyst at room temperature
A bimetallic catalyst and benzimidazole technology, which is applied in the field of photochemical organic synthesis, can solve the problems of difficult separation and purification of target compounds, non-recyclable catalysts, lowering reaction temperature, etc., and achieves high conversion rate and product selectivity. Wide and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for preparing benzimidazoles with a supported bimetallic catalyst at room temperature, comprising the preparation of the supported bimetallic catalyst and the preparation of the benzimidazoles:
[0035] The preparation method of supported bimetallic catalyst comprises the following steps:
[0036] Step (1) the oxide support TiO of 1.0g 2Disperse to 12.6mL Cu(NO 3 ) 2 With 6.2mL of 0.01M H 2 PtCl 6 In the mixed solution, stir at room temperature for 2 hours to obtain suspension A, then add 7mL of lysine solution with a concentration of 0.1M, continue stirring for 0.5 hours and adjust the pH of the suspension to 8-9 with a NaOH solution with a concentration of 0.3M. Prepare mixed solution B;
[0037] Step (2) Add 2 mL of NaBH with a concentration of 0.35M dropwise to the mixed solution B 4 solution, and then add 3.5mL of HCl solution with a concentration of 0.1M to obtain a mixture C, then leave the resulting mixture C at room temperature for 24 hours, pour...
Embodiment 2-4
[0054] Adopt the method in embodiment 1 to prepare Cu-Pt / TiO 2 Supported bimetallic catalysts.
[0055] Preparation of benzimidazole compounds: add 10mg Cu-Pt / TiO to a 25mL glass reactor 2 Supported bimetallic catalyst and 10mL organic solvent trifluorotoluene (embodiment 2), dichloromethane (embodiment 3) or cyclohexane (embodiment 4), then add 0.3mmol o-phenylenediamine and 0.6mmol ethanol, seal The glass reactor was turned on with a xenon lamp light source, and the reaction was terminated after 5 hours of light. The conversion rate and product structure of o-phenylenediamine were measured by gas chromatography and gas chromatography spectrometer respectively. The reaction results are shown in Table 3.
Embodiment 5
[0063] A method for preparing benzimidazoles with a supported bimetallic catalyst at room temperature, comprising the preparation of the supported bimetallic catalyst and the preparation of the benzimidazoles:
[0064] The preparation method of supported bimetallic catalyst comprises the following steps:
[0065] Step (1) 1.0g oxide support ZrO 2 Disperse to 12.6mL Cu(NO 3 ) 2 With 6.2mL of 0.01M H 2 PtCl 6 In the mixed solution, stir at room temperature for 2.5 hours to obtain suspension A, then add 3 mL of lysine solution with a concentration of 0.3M, continue stirring for 2 hours, and adjust the pH of suspension A to 8-9 with NaOH with a concentration of 0.1M. Prepare mixed solution B;
[0066] Step (2) Add 4.5mL of 0.2M NaBH dropwise to the mixed solution B 4 solution, and then add 1.8mL of HCl solution with a concentration of 0.2M to obtain a mixture C. The resulting mixture C was allowed to stand at room temperature for 36 hours, and the supernatant liquid was pour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com