Supported bimetallic catalyst for preparing aliphatic polycarbonate diol and preparation method thereof
A bimetallic catalyst and polycarbonate 2 technology are applied in the field of supported bimetallic catalysts and their preparation, which can solve the problems of inability to realize polycondensation reaction, low catalyst activity, difficult separation of catalysts, etc., and achieve good stability and repeated use. Good performance, catalytic effect, improved uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: KNO was sequentially added at room temperature 3 and Ca(NO 3 ) 2 Dissolve in a deionized aqueous solution with a pH value of 7 at a molar ratio of 1:1 to obtain a 0.05 g / mL bimetallic salt mixture solution, and then add 5 g of γ-Al with a particle size of 500 mesh 2 o 3 The carrier is added to the metal salt solution, stirred and impregnated for 4 hours to obtain a mixture of the supported bimetallic catalyst and water; the mixture of the supported bimetallic catalyst and water is filtered, and the filtered filter cake is dried in an oven at 120°C and dried Put it into a muffle furnace and bake at 600° C. for 5 hours to obtain a supported bimetallic catalyst.
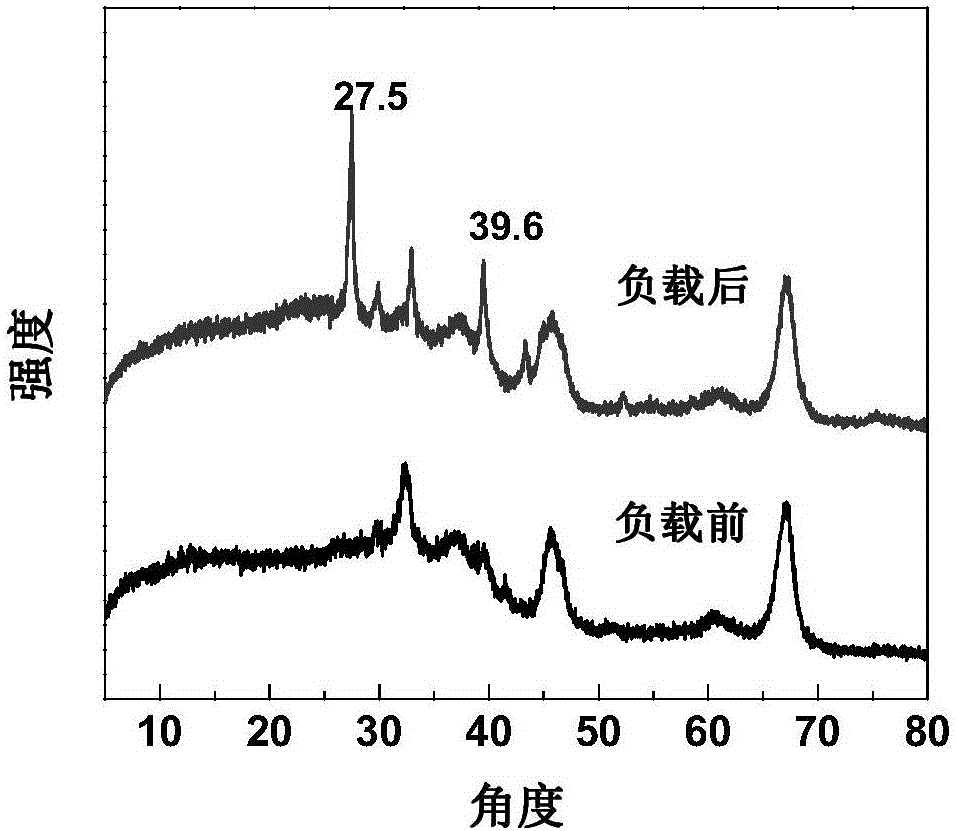
[0031] attached by figure 1 It can be seen that with the loading of the metal salt, the crystal structure of the catalyst has changed significantly. Through analysis, it can be found that the metal salt reacted with the support to form a new crystal structure, at 27.5 o , 39.6 o A new crystal pea...
Embodiment 2
[0038] Example 2: K at room temperature sequentially 2 CO 3 and Ca(NO 3 ) 2 Dissolve in a deionized aqueous solution with a pH value of 6 at a molar ratio of 1:1.1 to obtain a 0.01 g / mL bimetallic salt mixture solution, and then add 5 g of γ-Al with a particle size of 400 mesh 2 o 3 The carrier was added into the metal salt solution, stirred and impregnated for 2 hours to obtain a mixture of the supported bimetallic catalyst and water; the mixture of the supported bimetallic catalyst and water was filtered, and the filtered filter cake was dried in an oven at 80°C and dried Put it into a muffle furnace and bake at 550° C. for 4 hours to obtain a supported bimetallic catalyst.
[0039] The method for preparing aliphatic polycarbonate diols by using the supported bimetallic catalyst prepared by the above method, the specific operation process is as follows:
[0040] (1) In a reaction vessel equipped with a heating and stirring system, a reflux condensing system and a temper...
Embodiment 3
[0042] Example 3: KF and Ca(CH 3 COO) 2 Dissolve in a deionized aqueous solution with a pH value of 8 at a molar ratio of 1:0.5 to obtain a 0.01 g / mL bimetallic salt mixture solution, and then add 5 g of γ-Al with a particle size of 300 mesh 2 o 3 The carrier was added into the metal salt solution, stirred and impregnated for 8 hours to obtain a mixture of the supported bimetallic catalyst and water; the mixture of the supported bimetallic catalyst and water was filtered, and the filtered filter cake was dried in an oven at 160°C and dried Put it into a muffle furnace and bake at 800° C. for 2 hours to obtain a supported bimetallic catalyst.
[0043] The method for preparing aliphatic polycarbonate diols by using the supported bimetallic catalyst prepared by the above method, the specific operation process is as follows:
[0044] (1) In a reaction vessel equipped with a heating and stirring system, a reflux condensing system and a temperature measuring system, dimethyl carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com