UPLC-MS/MS method for measuring antiviral drug residuals in chicken
An antiviral drug, chicken technology, applied in the fields of analytical chemistry and food safety, can solve the problems of small scope of application, limited types, large matrix interference, etc., to reduce detection costs and organic solvent usage, save detection time, and simplify operations. effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
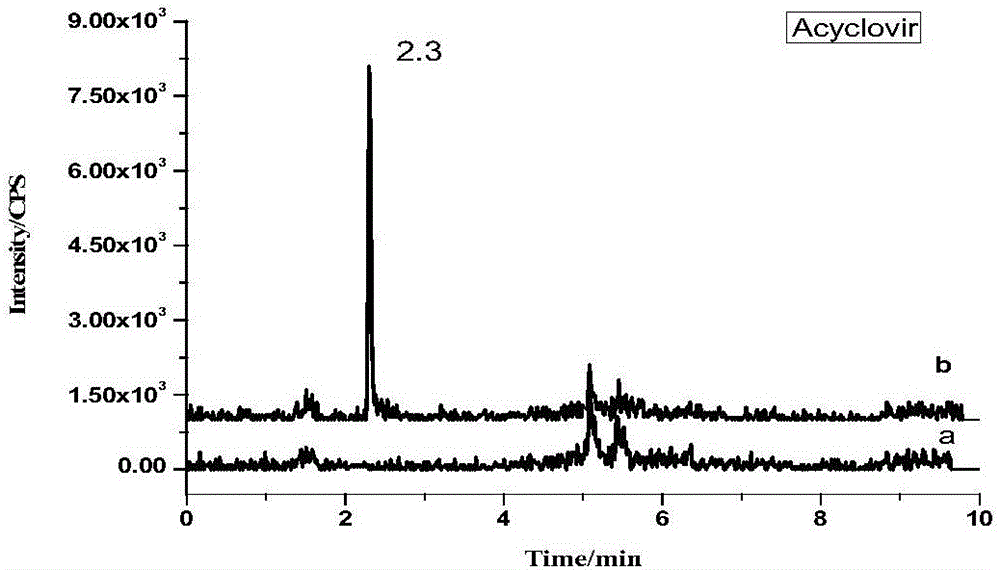
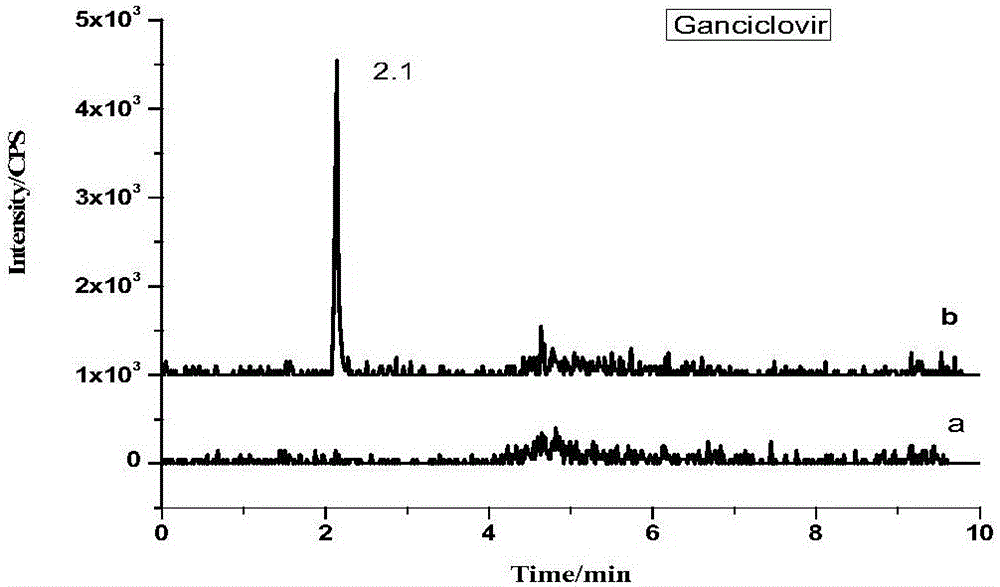
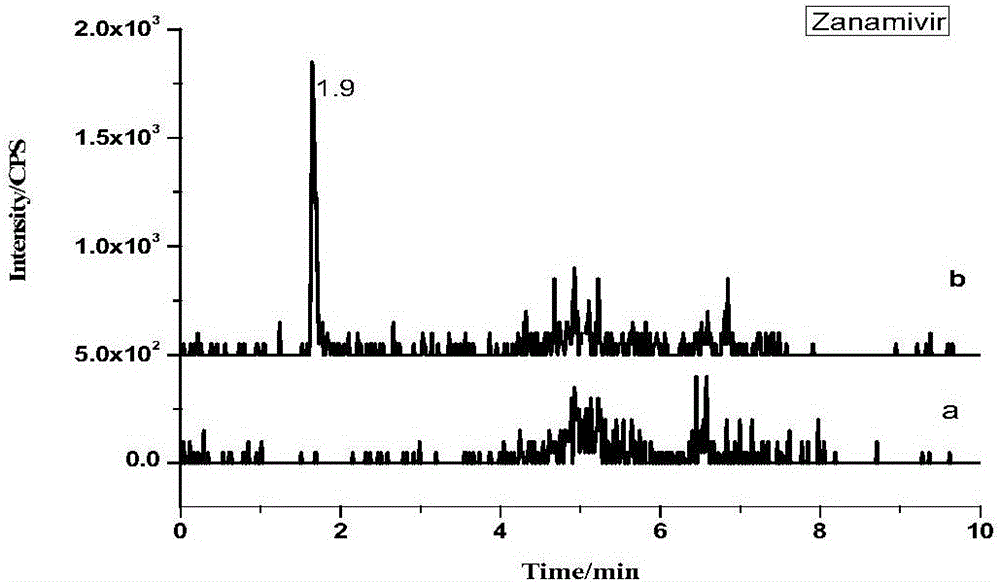
[0042] 1. Experimental principle
[0043] Under acidic conditions, use phosphatase to enzymatically hydrolyze the bound ribavirin, then extract ribavirin and other antiviral drugs with hydrochloric acid-methanol mixed solution, add water remover anhydrous sodium sulfate after separation , Purify impurities with C18 powder and PSA powder, determine with ultra-high performance liquid chromatography-tandem mass spectrometry, and quantify with isotope internal standard method.
[0044] 2. Materials and methods
[0045] 2.1 Test material
[0046] Standard products: amantadine, rimantadine, memantine, acyclovir, ganciclovir, zanamivir, lamivudine, arbidol, oseltamivir, imiquimod, Ribavirin, morpholinoguanidine and internal standards acyclovir-D4, amantadine-D5, memantine-D6, ribavirin-13C5 (purity>98.0%, purchased from Sigma Company).
[0047] Reagents: acetonitrile, methanol, formic acid (chromatographically pure, Merck, Germany); ethyl acetate, acetone, acetic acid, trichloroac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ion source temperature | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com