Preparation method of thermal-sensitive type catalyst used for asymmetric hydrogen transfer reaction
A catalyst and hydrogen transfer technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of separation and recovery of chiral metal complex catalysts that have not been well resolved, and achieve Overcome the difficult purification of products, the difficulty of catalyst recovery, high catalytic activity and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] A kind of preparation method of temperature-sensitive catalyst for asymmetric hydrogen transfer reaction
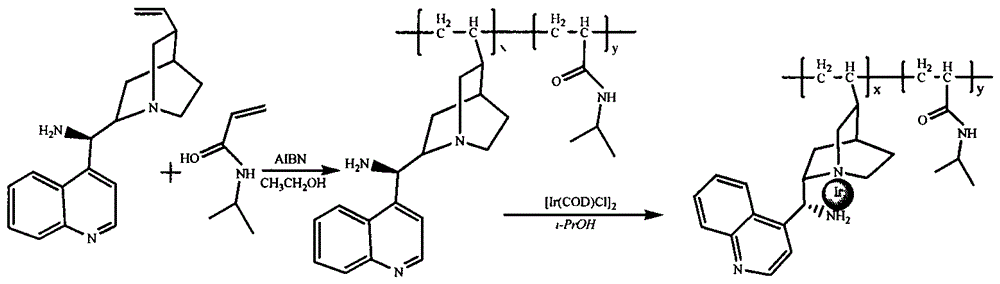
[0014] Add 0.293g of raw material 9-aminoepicincholine, 1.13g of N-isopropylacrylamide, 0.033g of initiator azobisisobutyronitrile and 17mL of absolute ethanol into the reaction vessel in sequence, stir to dissolve, and pass nitrogen gas to remove oxygen , heated to 70°C and reacted for 48h. After the reaction, the solvent was removed by rotary evaporation, 5mL of acetone was added, and 100ml of diethyl ether was used as a precipitant to precipitate the product, filtered by suction, and dried to obtain 9-aminoepicinchonine and N-isopropylpropene Copolymers of amides where x:y=1:10. Under a nitrogen atmosphere, 0.525 g of the copolymer, 0.021 g of 1,5-cyclooctadiene iridium chloride dimer, and 5 ml of isopropanol were added into a reaction vessel, and stirred at room temperature for 4 hours to obtain a catalyst.
Embodiment 2
[0016] A kind of preparation method of temperature-sensitive catalyst for asymmetric hydrogen transfer reaction
[0017] Add 0.293g of the raw material 9-aminoepichonine, 0.567g of N-isopropylacrylamide, 0.016g of the initiator azobisisobutyronitrile and 11ml of absolute ethanol into the reaction vessel in sequence, stir to dissolve, and pass through nitrogen to remove oxygen , heated to 70°C and reacted for 48h. After the reaction, the solvent was removed by rotary evaporation, 2.5mL of acetone was added, and 50ml of ether was used as a precipitating agent to precipitate the product, suction filtered, and dried to obtain 9-aminoepichonine and N-isopropyl Copolymers of acrylamide where x:y=1:5. Under a nitrogen atmosphere, 0.315 g of the copolymer, 0.021 g of 1,5-cyclooctadiene iridium chloride dimer, and 5 ml of isopropanol were added into a reaction vessel, and stirred at room temperature for 4 hours to obtain a catalyst.
Embodiment 3
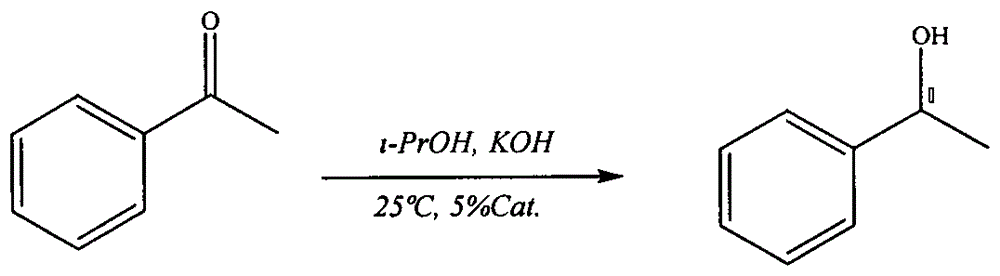
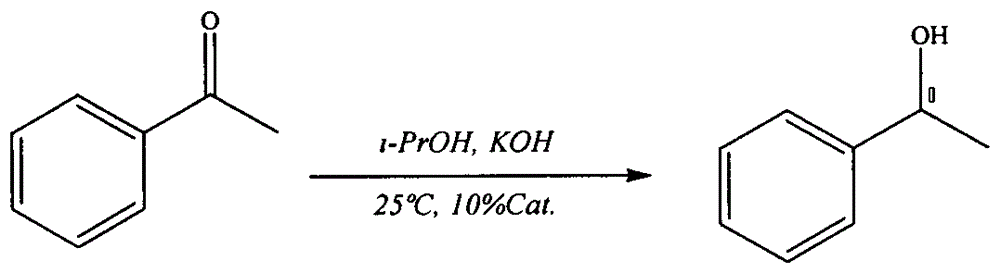
[0019] Dissolve 0.11 g of the prepared catalyst and 1.4 mg of potassium hydroxide in 5 ml of isopropanol, stir for 0.5 h, add 29 μL of acetophenone, and react at 25° C. for 180 min (see Table 1 for catalytic performance data). After the reaction, 6ml of water was added, and the temperature was raised to 40° C. to recover the catalyst by centrifugation.
[0020] The reaction formula is:
[0021]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com