Thiosemicarbazone 7-umbelliferone-8-aldehyde probe reagent as well as preparation and application thereof
A technology of hydroxycoumarin and thiosemicarbazide, applied in chemical instruments and methods, instruments, analytical materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: Synthesis of thiosemicarbazide 7-hydroxycoumarin-8-aldehyde probe reagent:
[0095] synthetic route:
[0096]
[0097] Specific steps:
[0098] 1. N 2 Under protection, add 120mL of dichloromethane, 7-hydroxycoumarin (9.3g, 57.3mmol) into a 250mL three-necked flask, stir to dissolve, then add acetic anhydride (11.7g, 114.6mmol), 7-8 drops of piperidine (0.3, g), reflux at room temperature, and react for 12 hours. After the reaction is completed, the solvent is distilled off under reduced pressure. After adding water, it is extracted with ethyl acetate. The ethyl acetate layer is washed with saturated brine, dried over sodium sulfate, filtered, and decompressed. The solvent was distilled off, and the crude product was separated by column chromatography using chloroform as the eluent to obtain 11.16 g of a white product, 7-acetoxycoumarin, with a yield of 95.4%. 1 H NMR (400MHz, CDCl 3 ), δ (ppm): 2.36 (s, 3H, CH 3 ), 6.41(d, 1H, J=9.6Hz, ArH), 7.07(...
Embodiment 2
[0102] The preparation methods of various reagents are:
[0103] 1. The preparation method of the probe reagent solution: weigh 26.3 mg of the probe reagent, dissolve it in DMF, and prepare a 100 mL solution with a concentration of 1 mM;
[0104] 2. Zn 2+ Reserve standard solution preparation method: weigh 74.4mg of analytically pure Zn(ClO 4 ) 2 , dissolved in acetonitrile, and prepared into 10 mL of a solution with a concentration of 20 mM; the preparation methods of the rest of the metal ions are the same as above;
[0105] 3. F- Stock solution preparation method: Weigh 52.8 mg of tetrabutylammonium fluoride, dissolve it in acetonitrile, and prepare 10 mL of a solution with a concentration of 20 mM. The preparation of other anion solutions is the same as above.
[0106] 4. 75% ethanol solution: add 75mL of absolute ethanol to 100mL with distilled water, mix well, and store at room temperature for later use;
[0107] 5. Phosphate buffer solution (D-hanks balanced salt s...
Embodiment 3
[0115] (1) Fluorescence spectrometry on Zn 2+ detection
[0116] Add (1mM, 100μL) to a 10mL volumetric flask, Zn 2+ (20mM, 0~100μL), with DMF / H 2 O (v / v, 1 / 4) was diluted to the mark, shaken up, and carried out fluorescence spectrum measurement;
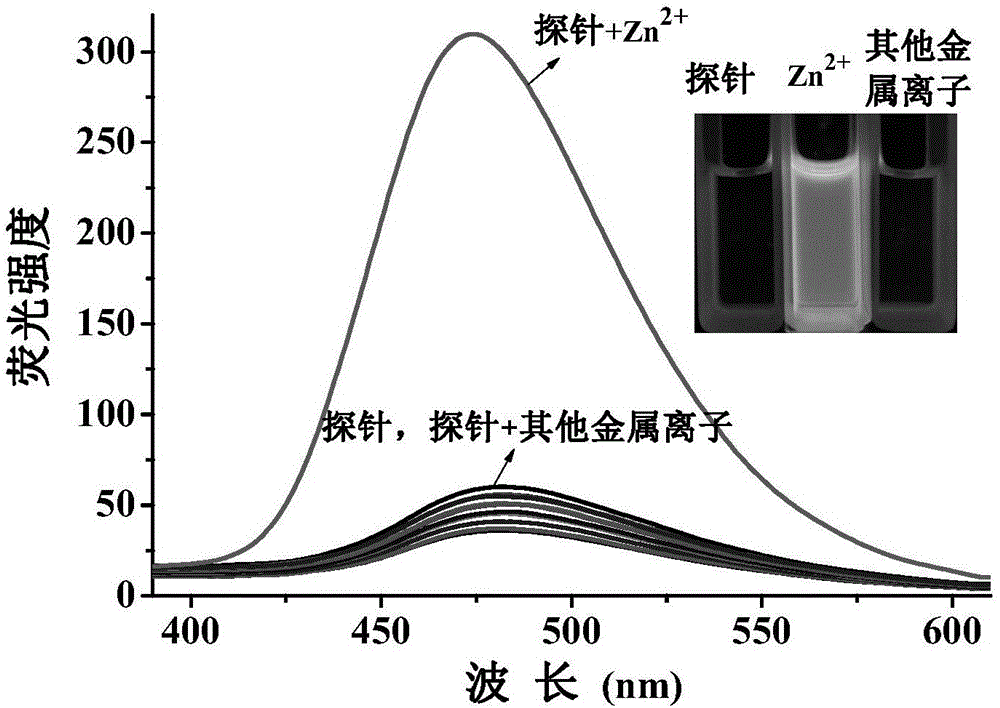
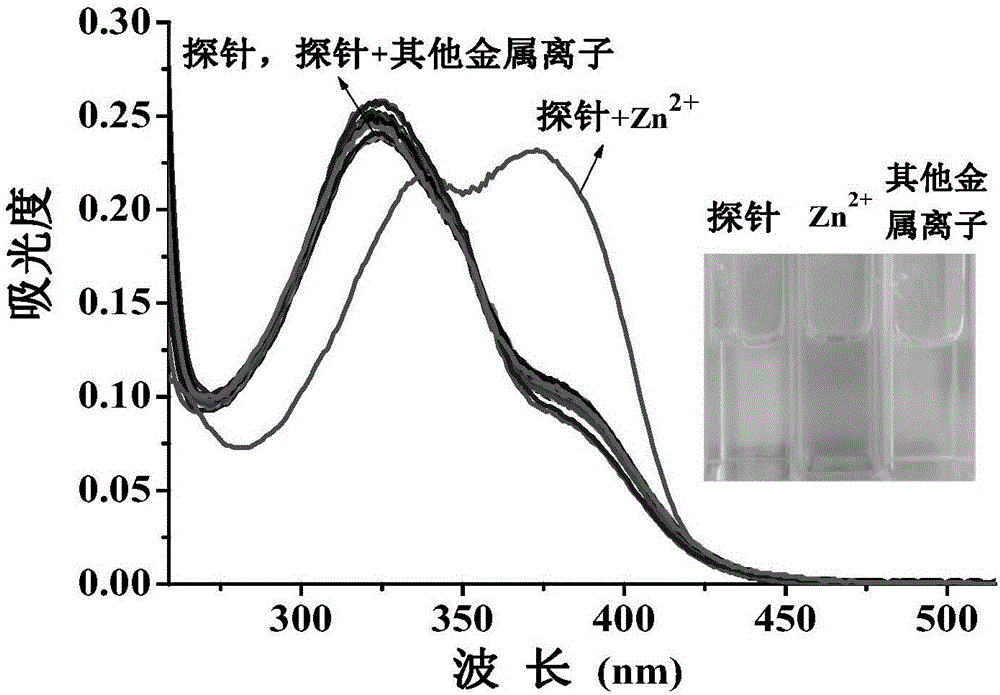
[0117] Set the fluorescence excitation wavelength to 355nm, add 3ml of probe reagent (10μM concentration) in DMF / H to a 1cm cuvette 2 The O (v / v, 1 / 4) solution is subjected to spectral scanning, and the probe reagent has fluorescence emission at a wavelength of 475nm. Add 20 μM metal ion Li + , Na + , K + , Mg 2+ , Ca 2+ , Ba 2+ , Hg 2+ ,Sr 2+ , Al 3+ , Cd 2+ , Ni 2+ ,Co 2+ , Pb 2+ , Fe 3+ , Cr 3+ , Ag 2+ , Cu 2+ , no obvious change in the fluorescence spectrum was observed, only Zn 2+ The addition of the probe reagent significantly enhanced the fluorescence peak at 475nm, such as figure 1 .
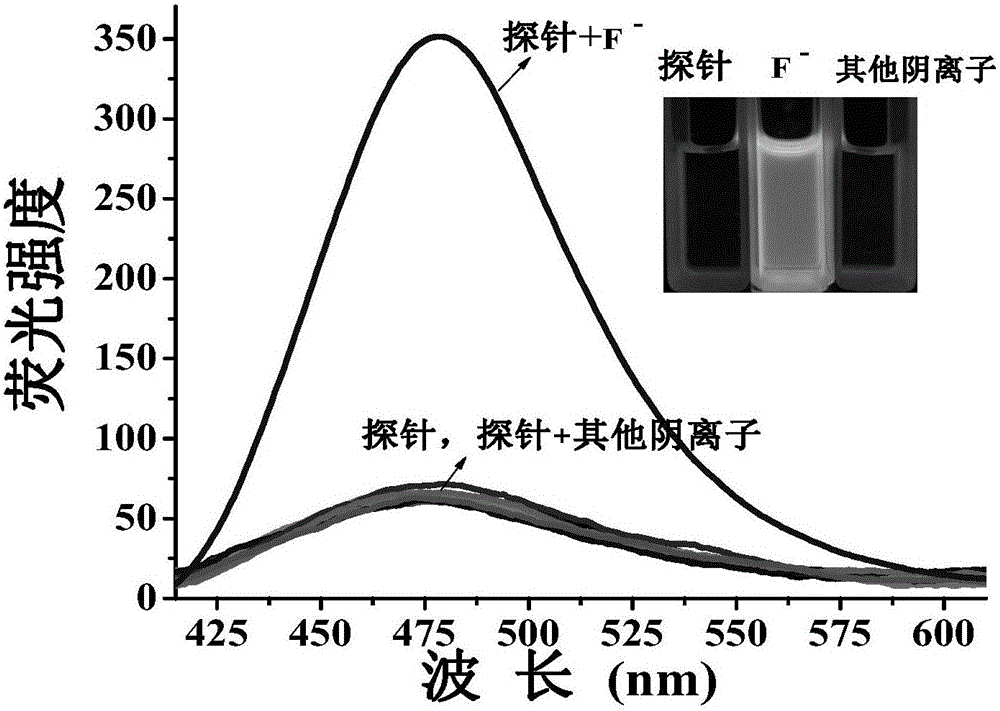
[0118] in DMF / H 2 O (v / v, 1 / 4) solution, with 410nm as the fluorescence excitation wavelength, gradually drop differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com