Protein or polypeptide drug-loaded tissue adhesive film and preparation method thereof
A sticking film and drug-loading technology, which is applied in the field of biomedicine, can solve the problems of failure to achieve clinical treatment effects, difficulty in effective control of sustained release, lack of drug delivery technology, etc., to achieve good degradable absorbability, direct drug delivery, high Biosecurity Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
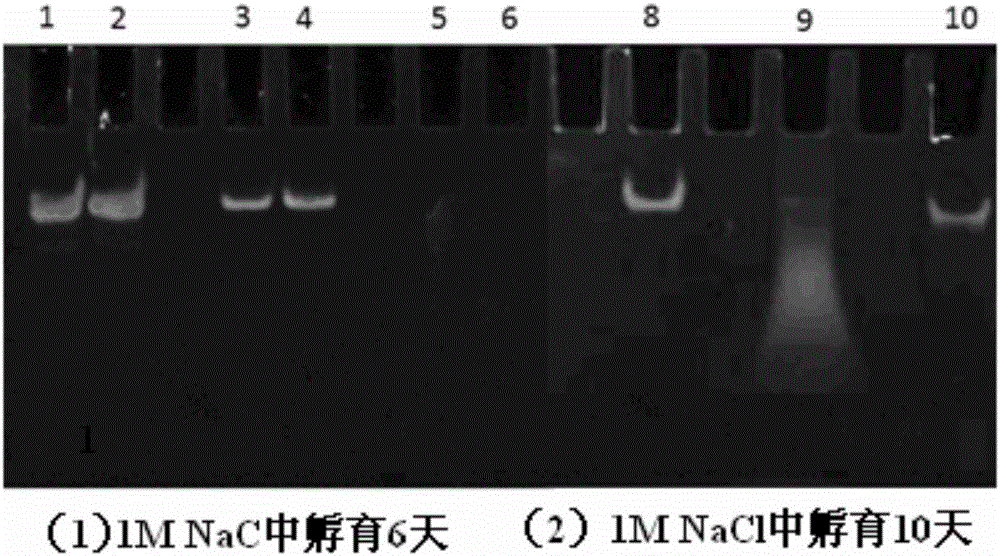
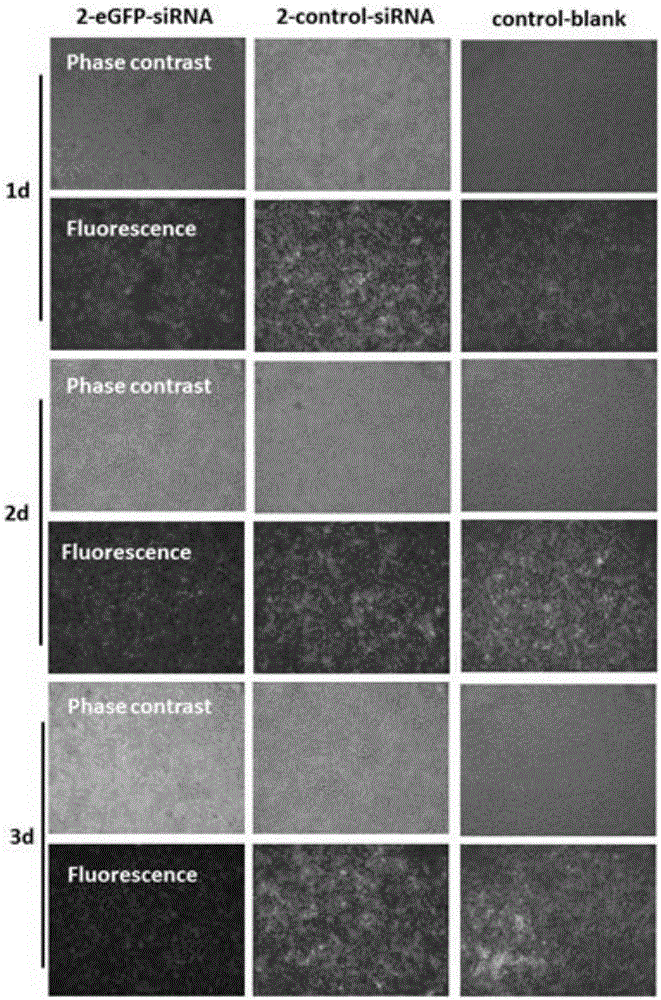
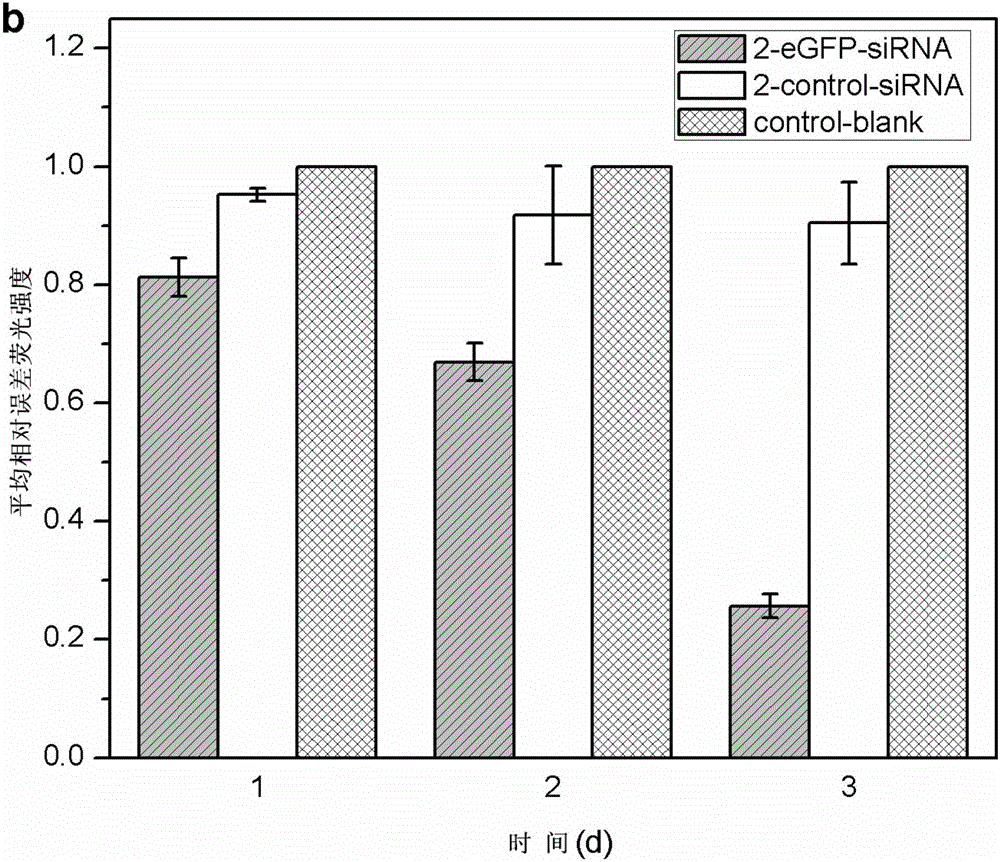
[0052] In a sterile workbench, prepare a 12 cm diameter petri dish with a built-in silicon wafer membrane of the same diameter (washed, sterilized and depyrogenated), and dry; respectively prepare solutions A (1 mg / kg) of materials with cationic groups. ml chitosan, 0.1mol / L acetic acid, 0.2mol / L NaCl, pH=4) and liquid B (1mg / ml carboxymethyl chitosan, 0.15mol / L NaCl, pH=6) with anionic group material Take part B liquid and add small interfering nucleic acid drug (eGFP-siRNA (sense: 5'-GGCACAAGCUGGAGUACAAUU-3'; antisense: 5'-UUGUACUCCAGCUUGUGCCUU-3', 20ug / mL) and cell targeting factor (hyaluronic acid, molecular weight Less than 20,000 Daltons, 10ug / mL) and targeting peptide (1×10 -4 mg / ml) was formulated into liquid C, and the amino acid sequence of the targeted polypeptide was: valine-glycine-valine-alanine-proline-glycine; the above solutions were respectively filled into high-pressure instantaneous spraying equipment, first Spray liquid C into the petri dish with high pre...
Embodiment 2
[0054] In a sterile workbench, prepare a plate of polymer material (washed, sterilized and depyrogenated), and dry; respectively prepare solutions A of materials with cationic groups (2 mg / ml chitosan, 0.1 mol / L acetic acid , 0.2mol / L NaCl, pH=4) and B solution (alginic acid of 2mg / ml, 60,000 to 80,000 molecular weight, 0.15mol / L NaCl, pH=6) with anionic group material is referred to as B solution; B solution Then add small interfering nucleic acid drug (same as Example 1, 20ug / mL) and cell targeting factor (hyaluronic acid, molecular weight is less than 20,000 Daltons, 10ug / mL), and then add targeting polypeptide (1 ×10 -4 mg / ml), the amino acid sequence of the targeted polypeptide is: isoleucine-lysine-valine-alanine-valine; first immerse the plate in solution A for 20 minutes, take it out into the cleaning solution, wash and dry; Then immerse the plate in solution B for 30 minutes, take it out, wash and dry it with cleaning solution, do this alternately 160 times, and fina...
Embodiment 3
[0056] In a sterile workbench, prepare a plate of polymer material (washed, sterilized and depyrogenated), and dry; respectively prepare solutions A of materials with cationic groups (2 mg / ml chitosan, 0.1 mol / L acetic acid , 0.2mol / L NaCl, pH=4) and B solution with anionic group material (2mg / ml hyaluronic acid, 0.15mol / L NaCl, pH=6) referred to as B solution; Nucleic acid cationic liposome (cationic lipid preparation method refers to "gene transfection mediated by anionic liposome-cationic liposome complex", Journal of Pharmaceutical Practice, 2011 (29): 4, small interfering nucleic acid is the same as Example 1 , small interfering nucleic acid cationic liposome addition amount is 0.5mg / ml, small nucleic acid drug load 21.7%) stir evenly and targeting polypeptide (1 * 10 -4 mg / ml), the amino acid sequence of the targeted polypeptide is: arginine-glycine-aspartic acid; first immerse the plate in solution A for 20 minutes, take it out into the cleaning solution, wash and dry; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com