Recycling method and application of L-methyldopa intermediate
A kind of technology of methyldopa intermediate and L-methyldopa, which is applied in the field of recovery of L-methyldopa intermediate, can solve problems such as no literature report, and achieves strong operability, easy process implementation and high efficiency. Good production and controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
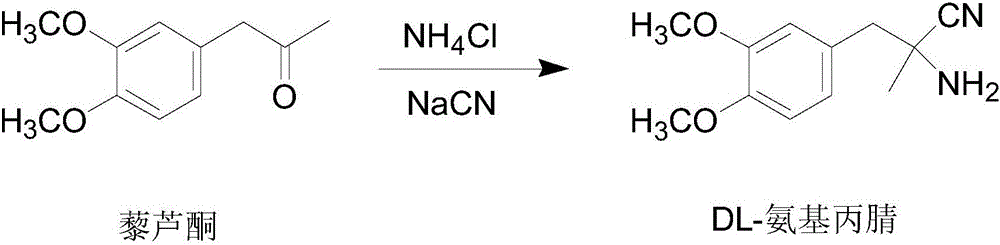
[0039] 1) Synthesis of DL-aminopropionitrile
[0040] Dissolve 154.5g (3.15mol) of 96% to 98% sodium cyanide and 163g (3.05mol) of ammonium chloride in 2200ml (26.8mol) of 12.2mol / L ammonia solution at room temperature, heat to 55°C and stir rapidly Quickly add 582.6g (3mol) veratrone, stir for another 1h, then cool down to below 20°C within 1.5h, fine particle products start to precipitate, keep stirring for 2h, cool down to 0°C, filter with suction, the filtrate is DL-amino The mother liquor of propionitrile synthesis, the filter cake was washed with 800ml of ice water, and dried to obtain 618.42g (2.81mol) of DL-aminopropionitrile, with a molar yield of 93.7%, and a melting point of 85°C to 87°C. The obtained DL-aminopropionitrile could be Purification directly resolved.
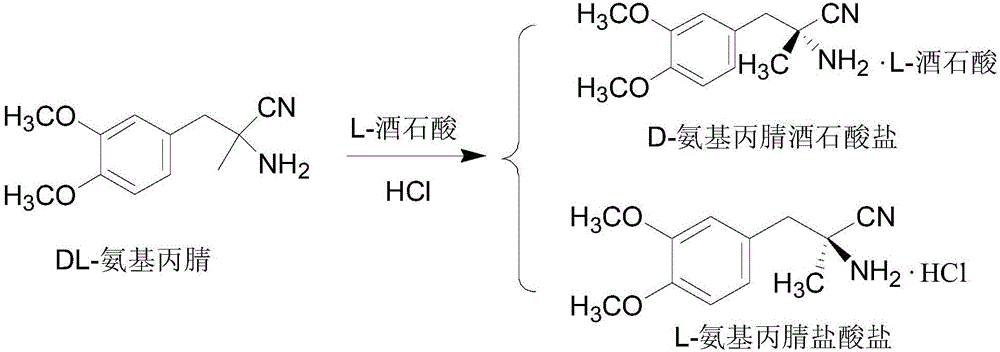
[0041] 2) Resolution of DL-aminopropionitrile
[0042] 125g of D-tartaric acid (0.83mol) was dissolved in 500ml of water, and the pH was adjusted to 8 with sodium hydroxide to obtain a D-tartrate disodi...
Embodiment 1
[0051] Get the mother liquor 262.5ml that step 4) obtains (containing 3-(3,4-dimethoxyphenyl)-2-methylalanine hydrochloride 10.67g), add ammonium sulfate 32.8g (volume weight ratio mother liquor : Salt = 8: 1), carry out 60 ℃, vacuum-0.08mpa decompression distillation, distill 168ml of water (COD: 798mg / L), a small amount of crystals precipitate, cool down to 0 ℃, filter, add 10ml of ice water (0 ℃ ) was washed to obtain a solid, which was dried to obtain 9.7g, the recovery rate of 3-(3,4-dimethoxyphenyl)-2-methylalanine hydrochloride was 90.9%, and the HPLC purity was 98.3%; the mother liquor continued to distill , distilled 30ml of water (COD: 839mg / L), a large number of crystals precipitated, cooled to 0°C, filtered to obtain a solid, dried to obtain 44.1g, containing COD: 1.2g, mother liquor 14ml, COD: 40936mg / L.
Embodiment 2
[0053] Get the mother liquor 262.5ml that step 4) obtains (containing 3-(3,4-dimethoxyphenyl)-2-methylalanine hydrochloride 10.67g), add ammonium chloride 21.8g (volume weight ratio Mother liquor: salt = 12:1), carry out 60°C, vacuum-0.08mpa reduced-pressure distillation, distill 200ml of water (COD: 796mg / L), cool to 0°C, filter to obtain a solid, dry to obtain 10.1g, 3-( The recovery rate of 3,4-dimethoxyphenyl)-2-methylalanine hydrochloride is 95%, and the HPLC purity: 98.3%; the mother liquor continues to distill, and 34ml of water is distilled, a large number of crystals are precipitated, and the temperature is lowered to 0°C , filtered to obtain a solid, dried to obtain 34.2g, containing COD: 0.98g, mother liquor 10.8ml, COD: 23578mg / L
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com