A kind of polytriazole with intrinsic flame retardancy and its preparation method and application
An intrinsic flame retardant and polytriazole technology, applied in the fields of polymer chemistry and materials science, achieves the effects of high degree of freedom, simplified experimental operation, and great advantages in flame retardant performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
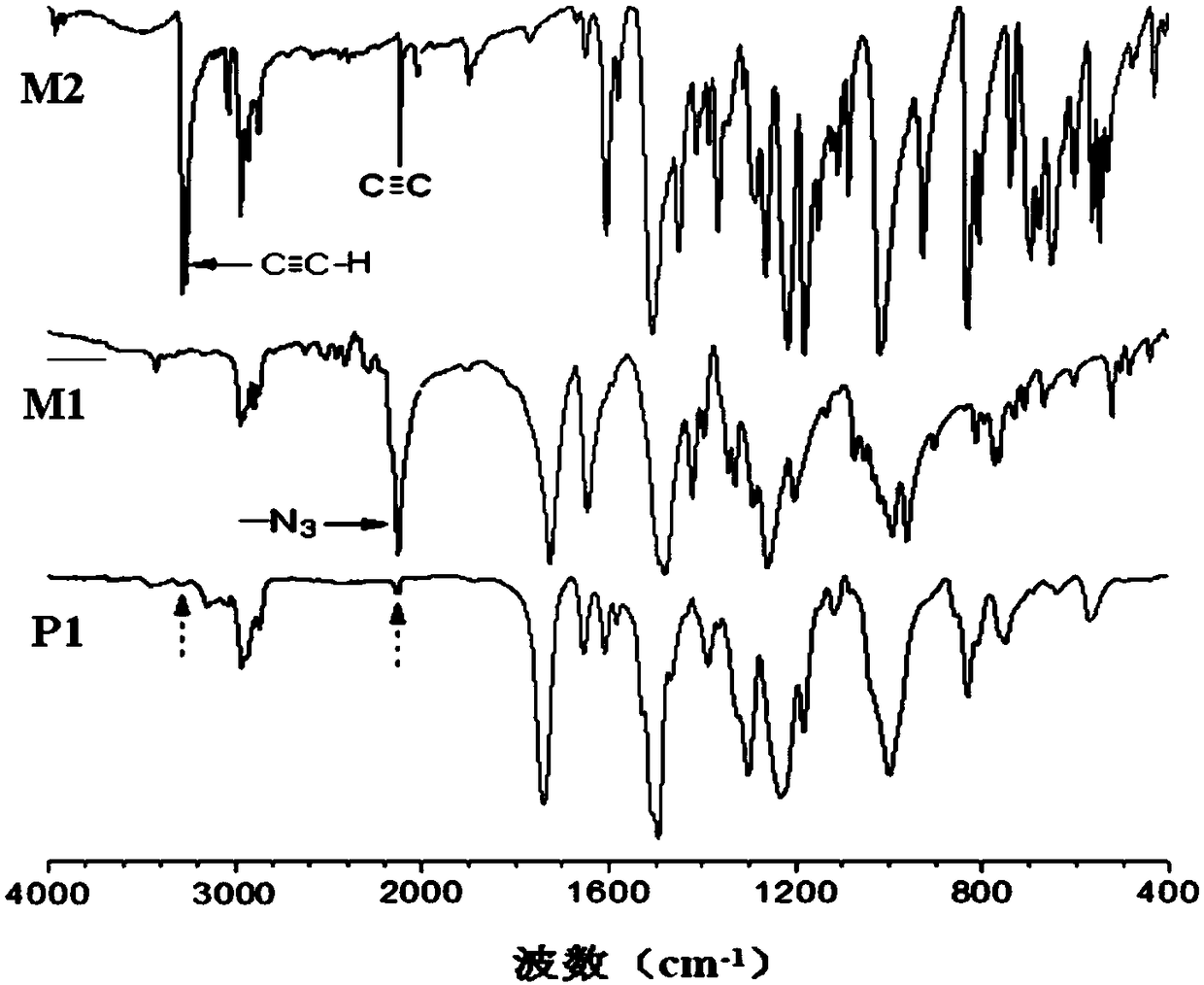
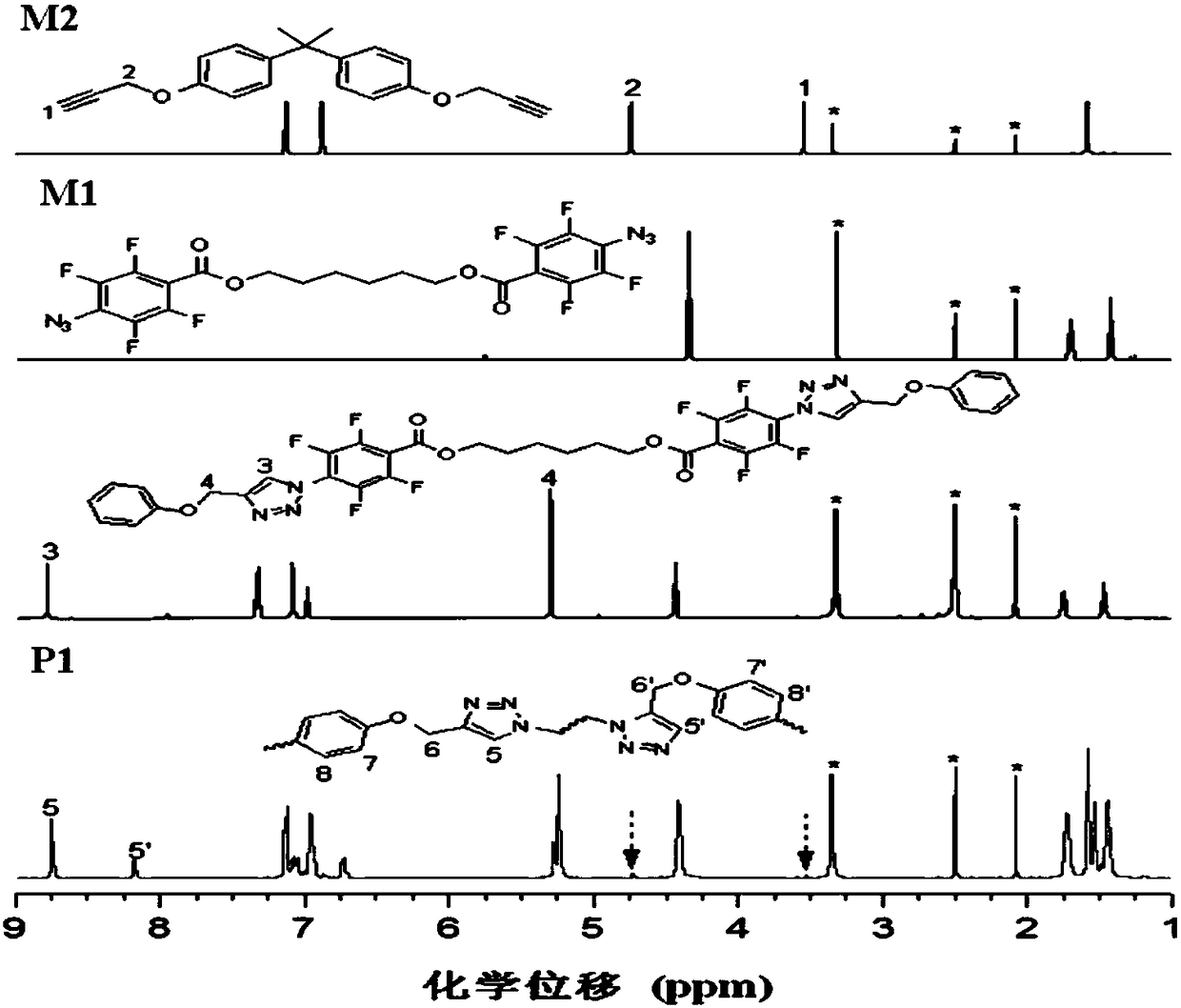
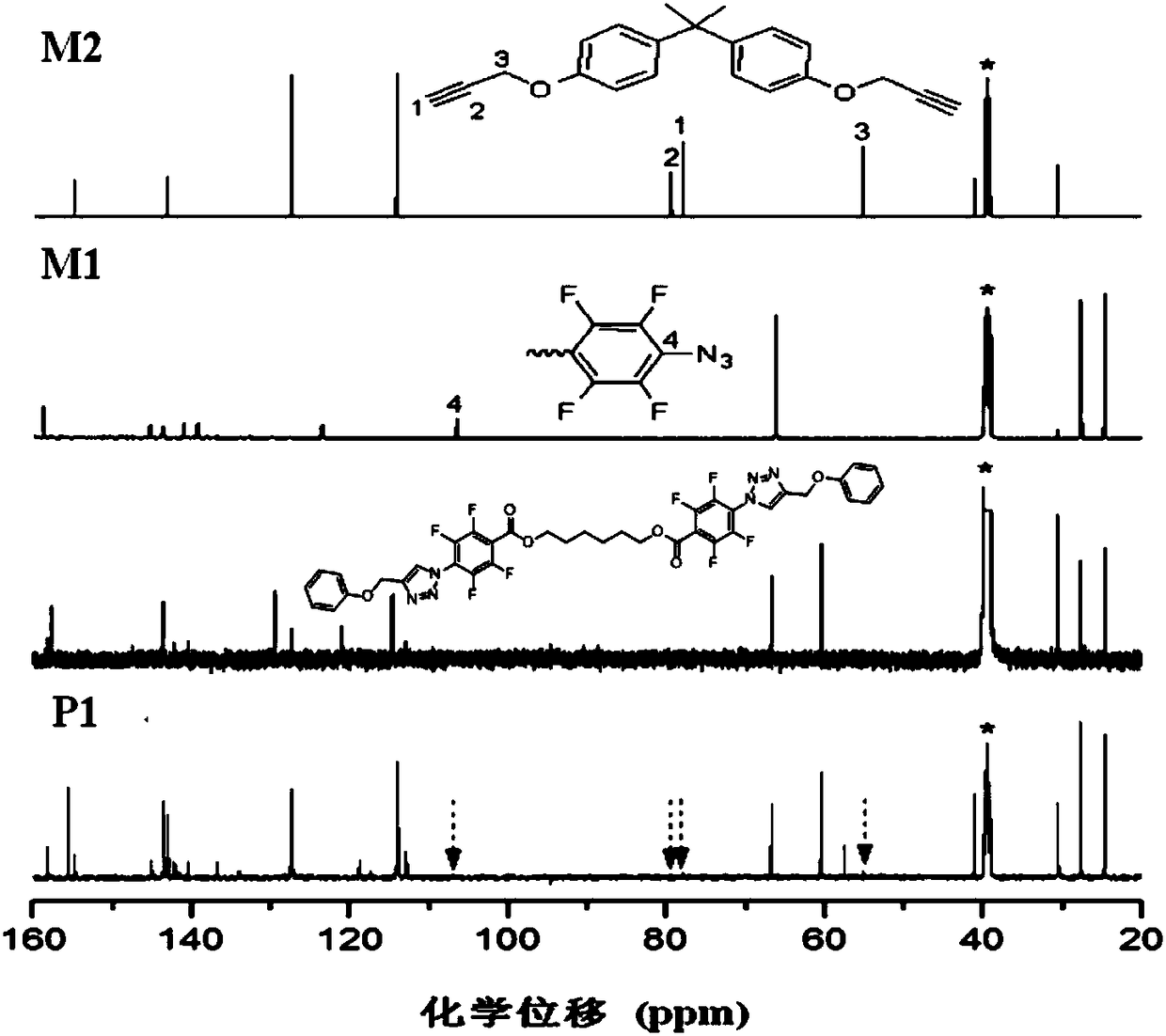
[0050] A kind of polytriazole, its structural formula is as shown in P1:
[0051]
[0052] The reaction equation is as formula (1):
[0053]
[0054] Among them, the monomer M2 is synthesized according to the method reported in the published literature (J.Fluorine Chem.2008, 129, 1003), and the synthesis method of the monomer M1 is as follows:
[0055] The reaction equation of monomer M1 is as formula (2):
[0056]
[0057] Among them, the molecule 2,1,6-hexanediol can be purchased from the market, in this example, it is purchased from TCI Company.
[0058] The preparation steps of monomer M1 are as follows:
[0059] Add 3.46mL of pentafluorobenzoyl chloride to 20mL of dichloromethane, add a mixed solution of 25mL of dichloromethane, 1.18g of 1,6-hexanediol, and 3.6mL of triethylamine dropwise to the above 20mL of dichloromethane The mixed solution was stirred at room temperature for three hours. After filtration, the filtrate was concentrated to obtain molecule 3...
Embodiment 2
[0066] A kind of polytriazole, its structural formula is as shown in P2:
[0067]
[0068] The reaction equation is as formula (3):
[0069]
[0070]
[0071] Wherein, the synthetic method of monomer M1, M2 is the same as embodiment 1.
[0072] The preparation steps of P2 are as follows:
[0073] 99.4 mL of monomer M1 and 57.2 mg of monomer M2 were added to a 10 mL thick-walled polymerization tube. The polymerization tube was placed in an oil bath at 110° C. and stirred for 2 h. Cool, add 4 mL of chloroform to dissolve, then add it dropwise into 500r / min stirred methanol, let stand, filter, and dry to obtain polytriazole P2.
[0074] After determination and analysis, the yield of polytriazole P2 was 81%, the weight average molecular weight was 100,000, and the molecular weight distribution was 2.62.
Embodiment 3
[0076] A kind of polytriazole, its structural formula is as shown in P3:
[0077]
[0078] The reaction equation is as formula (4):
[0079]
[0080] Wherein, the synthetic method of monomer M2 is the same as embodiment 1, and the reaction equation of monomer M3 is as formula (five);
[0081]
[0082] Among them, molecule 1 was purchased from TCI Company, and molecule 3 was purchased from J&K Company.
[0083] The preparation steps of monomer M3 are as follows:
[0084] 5.53g of pentafluorobenzoyl chloride was added to 25mL of dichloromethane, and a mixed solution of 25mL of dichloromethane, 2.28g of bisphenol A, and 2.83g of triethylamine was added dropwise to the above-mentioned 25mL of dichloromethane mixed solution, Stir at room temperature for three hours. After filtration, the filtrate was concentrated to obtain molecule 4.
[0085] Add 3.08g of molecule 4 and 0.78g of sodium azide into 80mL of acetone-water mixed solvent (volume ratio: 3:1), and stir overni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com