Creatinase mutant with improved heat stability
A creatine hydrolase and mutant technology, applied in the field of enzyme engineering, can solve the problems of decreased enzyme activity and unsatisfactory thermal stability, and achieve the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The acquisition of embodiment 1 high thermostability mutant strain
[0014] Using the site-directed mutagenesis kit (TaKaRa), design multiple pairs of primers, and use the vector pET20J (highly efficient heterologous expression and application analysis of Arthrobacter tobacco creatinase [J]. Food and Biotechnology Journal) to pair the 368th and 368th primers Saturation mutations were performed at two sites at position 195. The nucleotide sequence encoding wild creatine hydrolase is shown in SEQ ID NO.6.
[0015] The PCR reaction conditions were 98°C for 3min, 34 cycles (98°C for 3min, 58°C for 30S, 72°C for 1min30S), and 72°C for 10min. PCR amplification system: template 1 μl, upstream and downstream primers 1 μl each, 2x PrimeStar 24 μl, sterilized double distilled water 24 μl. After PCR, add 5 μl of FD Buffer and 1 μl of DpnI to digest for 1 hour.
[0016] The plasmid containing the gene encoding the mutant was transformed into E.coli BL21, and the selected transfo...
Embodiment 2
[0017] The purification of embodiment 2 creatine hydrolase
[0018] After crushing Escherichia coli, carry out ammonium sulfate precipitation, select the precipitation with 55%--75% ammonium sulfate saturation, dissolve the protein precipitate with a small amount of phosphate buffer (pH 7.0), and dialyze for 24 hours to remove ammonium sulfate in the enzyme solution. According to the isoelectric point properties of creatine hydrolase, the QFF column was selected for ion exchange purification. The QFF column was equilibrated with phosphate buffer for 30 minutes, the crude enzyme solution was injected into the purification column, and the target protein was eluted with phosphate buffer containing 1M NaCl.
Embodiment 3
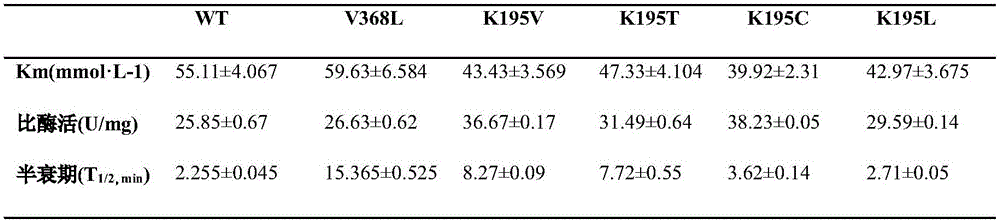
[0019] The property determination of the pure enzyme liquid of embodiment 3 mutants
[0020] Dilute each of the purified creatine hydrolase mutants, measure the enzyme activity of the mutants at 50°C for different times and calculate their half-life (t 1 / 2 , min), five mutants V368L, K195V, K195T, K195C, K195L were found to be 6.9, 3.7, 3.4, 1.6, 1.2 times higher than WT (as shown in Table 1). In addition, K195C's K m Compared with WT, the specific enzyme activity of K195V and K195C increased by 41.9% and 47.9%, respectively.
[0021] Table 1 Enzymatic Properties
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com