Application of anti-corrosion electrode in improving the selectivity and yield of organic electrosynthesis reaction
A synthesis reaction and selectivity technology, applied in the direction of electrolytic organic production, electrolytic organic material coating, electrodes, etc., can solve the problems of low adhesion, low reaction efficiency, easy peeling of polyaniline protective film, etc., to avoid corrosion and slag , good selectivity, and increased capture and response capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Surface pretreatment of substrate electrodes:
[0027] Use an ultrasonic cleaner to clean the surface of the graphite substrate electrode to remove excess grease, dust and other impurities, then put it in an ethanol solution (95% concentration) for cleaning, take it out and dry it naturally, and keep it for later use.
[0028] (2) Preparation of the base electrode
[0029] Add 0.01 g of dopamine and 0.01 g of trometamol into 50 mL of distilled water, and then put them in an ultrasonic cleaner for 10 minutes of sonication to dissolve and disperse. After being uniformly dispersed, the pretreated electrodes were soaked in the solution for 4 hours and then dried.
[0030] (3) Preparation of electrolyte:
[0031] Take 25mL of 0.1mol / L aniline aqueous solution and 25mL of 1.0mol / L sulfuric acid solution into a 100mL beaker, and let it stand for later use.
[0032] (4) Coating on the surface of the base electrode:
[0033] At room temperature, in the electrolyte, the p...
Embodiment 2
[0045] (1) The surface pretreatment of the substrate electrode is the same as in Example 1.
[0046] (2) Preparation of the transition layer on the surface of the base electrode
[0047] Add 0.001g of octadecylamine into 50mL of distilled water, place it in an ultrasonic cleaner for 20 minutes of ultrasonic treatment to dissolve and disperse. After being uniformly dispersed, soak the pretreated electrode in the solution for 2 hours. Take it out to dry.
[0048] (3) Electrolyte preparation:
[0049]Take 25mL of 0.2mol / L pyrrole solution and 50mL of 1.0mol / L hydrochloric acid solution into a 100ml beaker and mix well.
[0050] (4) Coating on the surface of the base electrode:
[0051] At room temperature, in the electrolyte, the polypyrrole film is electroplated on the surface of the carbon electrode by cyclic voltammetry, wherein the carbon electrode is the working electrode, the platinum electrode is the counter electrode, the calomel electrode is the reference electrode, ...
Embodiment 3
[0060] (1) The surface pretreatment of the substrate electrode is the same as before.
[0061] (2) Preparation of the transition layer on the surface of the base electrode:
[0062] Add 0.05g of silane coupling agent to 50mL of distilled water, and put it in ultrasonic treatment for 10min to dissolve and disperse. After being uniformly dispersed, soak the treated electrode in the solution for 4 hours, take it out and dry it for later use.
[0063] (3) Electrolyte preparation:
[0064] Add 30 mL of 0.5 mol / L thiophene solution and 25 mL of 0.5 mol / L sulfuric acid solution into a 100 mL beaker and mix well.
[0065] (4) Electrode surface coating:
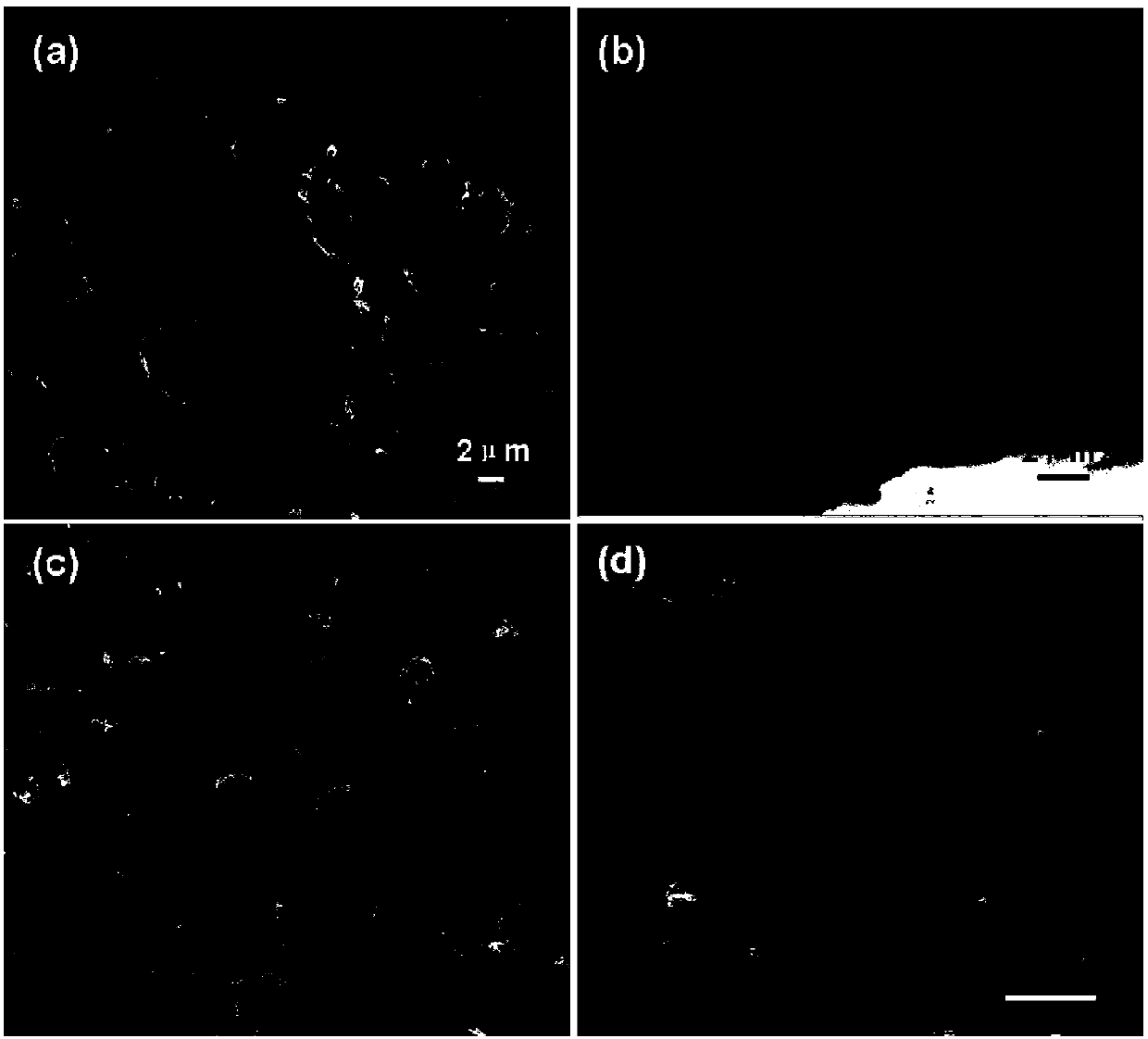
[0066] At room temperature, in the electrolyte solution, the nickel electrode is used as the anode, and the platinum electrode is used as the cathode. The system is electrolyzed with electricity. The electrolysis voltage is 3.0V and the electrolysis time is 2.0h. The surface morphology of the formed conductive polymer film is as fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com