A drug-carrying system capable of specific drug release at tumor sites and its preparation method
A drug-carrying system and specific technology, which can be used in anti-tumor drugs, pharmaceutical formulations, medical preparations with inactive ingredients, etc. problems, to achieve the effect of improving bioavailability, obvious toxicity, and high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
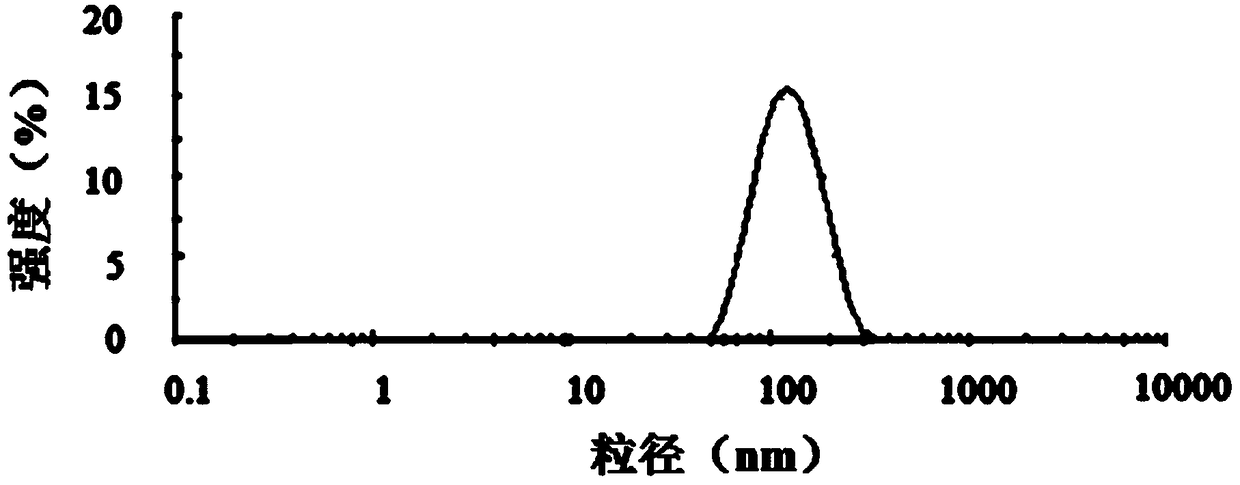
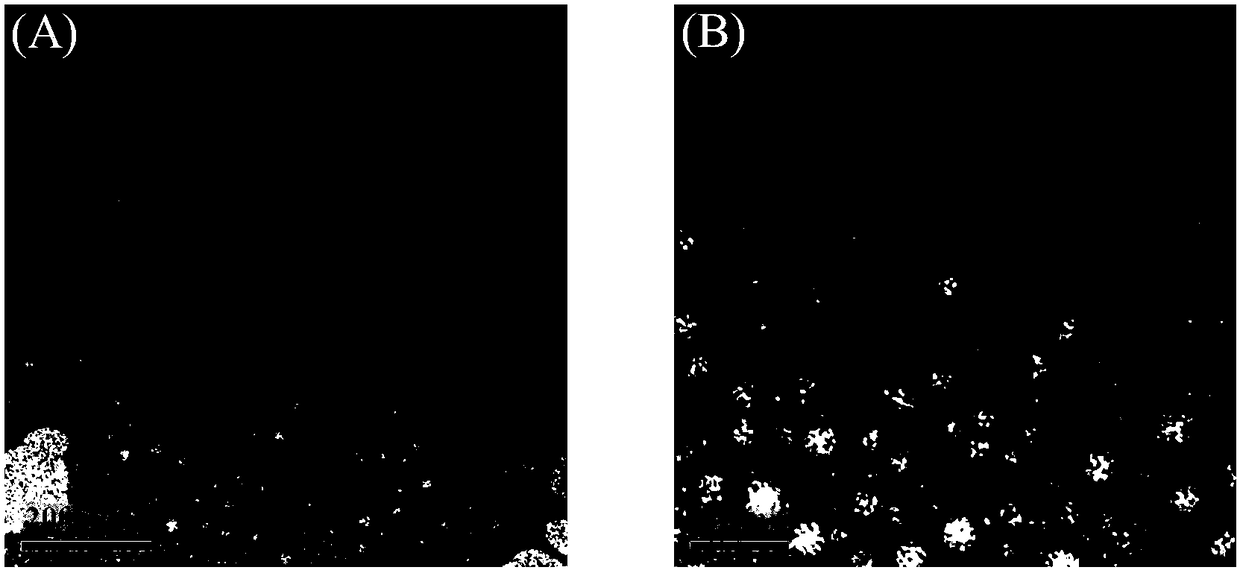
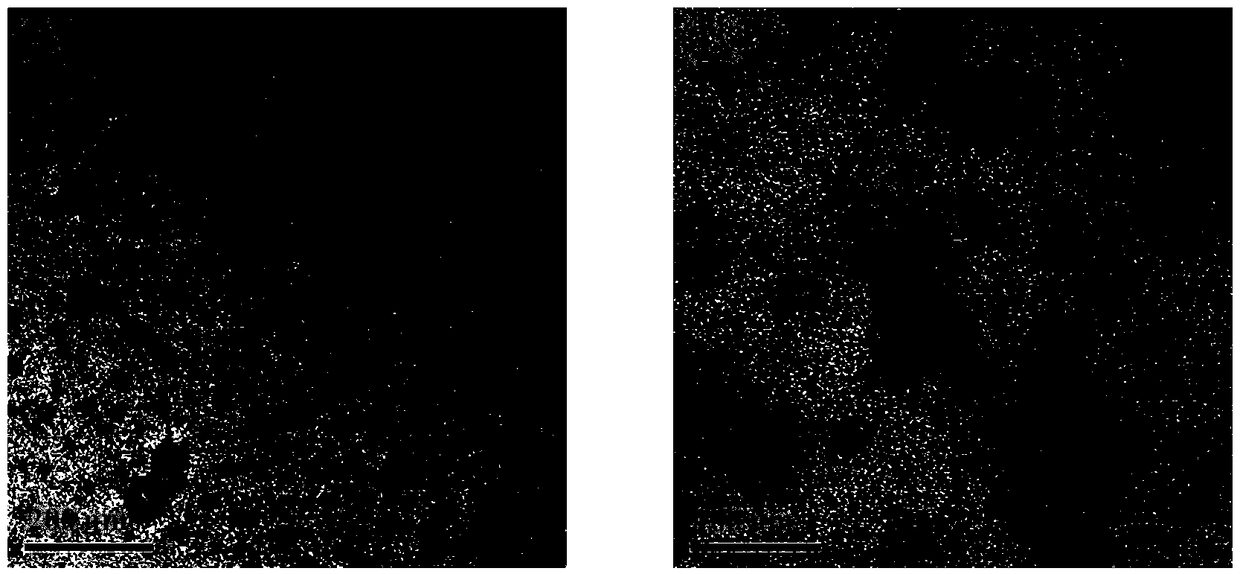
Image
Examples
Embodiment 1
[0043] 1. Preparation of cystamine-modified hyaluronic acid:
[0044] Dissolve 0.5 mmol of hyaluronic acid (HA) in 50 mL of 0.01 M, pH 7.4 phosphate buffered saline (PBS), then add 2 mmol of 1-ethyl-(3-dimethylaminopropyl)carbodiimide (EDC) and 0.4mmol N-hydroxysuccinimide (NHS), reacted at room temperature for 15 minutes; then added 5mmol cystamine (CYS), and continued to react at room temperature for 4 hours; In the bag, dialyze in 0.1M sodium chloride solution and deionized water for 1 day and 2 days respectively, and freeze-dry to obtain the cystamine-modified hyaluronic acid intermediate.
[0045] 2. Preparation of deoxycholic acid activated ester:
[0046] 1mmol of deoxycholic acid, 1.2mmol of dicyclohexylcarbodiimide (DDC), and 1.2mmol of N-hydroxysuccinimide (NHS) were dissolved in tetrahydrofuran (THF), reacted for 30 minutes under ice-cooling, and then React at room temperature for 12 hours; remove the precipitate by suction filtration, precipitate with excess ice ...
Embodiment 2
[0084] 1. Preparation of cystamine-modified hyaluronic acid:
[0085] As described in Example 1.
[0086] 2. Preparation of deoxycholic acid activated ester:
[0087] As described in Example 1.
[0088] 3. Preparation of deoxycholic acid-cystamine-hyaluronic acid graft copolymer:
[0089] As described in Example 1.
[0090] 4. Preparation of drug-loaded polymer micelles (paclitaxel (PTX)-deoxycholic acid-cystamine-hyaluronic acid micelles):
[0091] As described in Example 1.
[0092] 5. Preparation of the drug-loading system (calcium carbonate nanoshell-PTX-deoxycholic acid-cystamine-hyaluronic acid micelles):
[0093] Measure 10 mL of drug-loaded polymer micelles (paclitaxel (PTX)-deoxycholic acid-cystamine-hyaluronic acid micelles), adjust the pH of the drug-loaded micelles to 7.0, and then stir at room temperature at 400 rpm ;
[0094] According to the molar weight of carboxyl groups on the surface of the micelles (the total molar weight of carboxyl groups on hyalur...
Embodiment 3
[0097] 1. Preparation of cystamine-modified hyaluronic acid:
[0098] As described in Example 1.
[0099] 2. Preparation of octanoic acid-cystamine-hyaluronic acid graft copolymer:
[0100] According to the molar ratio of octanoic acid to 1-ethyl-(3-dimethylaminopropyl) carbodiimide and hyaluronic acid modified by cystamine as 5:5:1, feed into formamide and react at room temperature After 24 hours, the reaction was terminated, dialyzed in a mixed solution of ethanol and water with a volume ratio of 1:1, and in deionized water for 1 day and 2 days respectively, and freeze-dried to obtain octanoic acid-cystamine-hyaluronic acid grafted branch copolymers.
[0101] 3. Preparation of drug-loaded polymer micelles (paclitaxel (PTX)-octanoic acid-cystamine-hyaluronic acid micelles):
[0102] Dissolve 60 mg of caprylic acid-cystamine-hyaluronic acid graft copolymer in 10 mL of deionized water and stir at room temperature for 15 minutes to obtain polymer micelles (caprylic acid-cysta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com