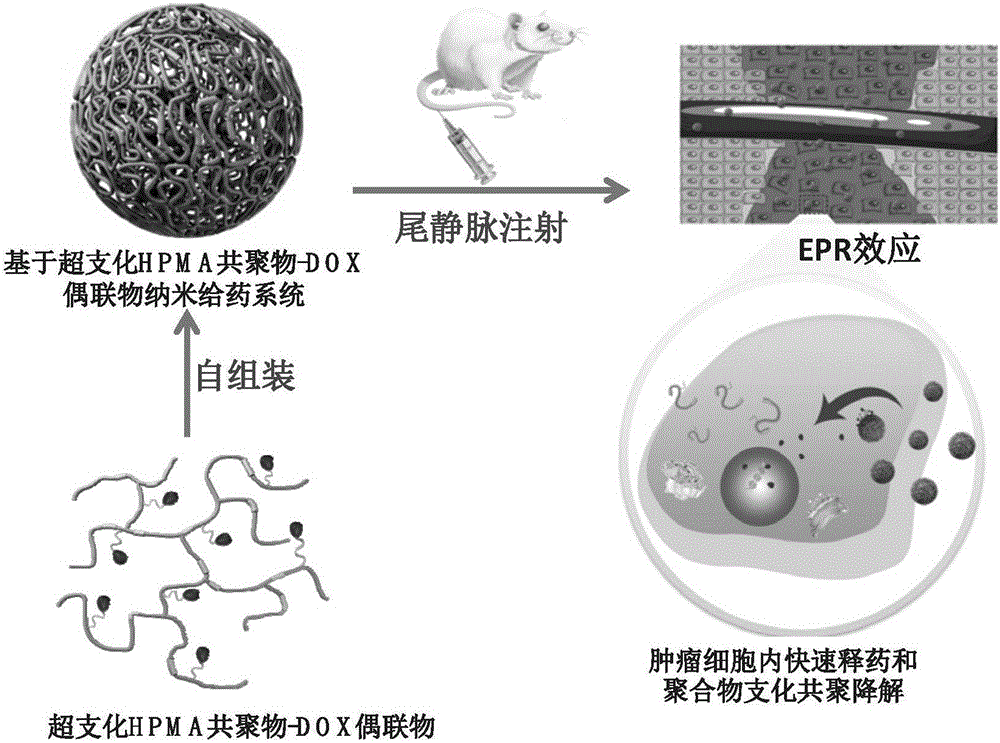
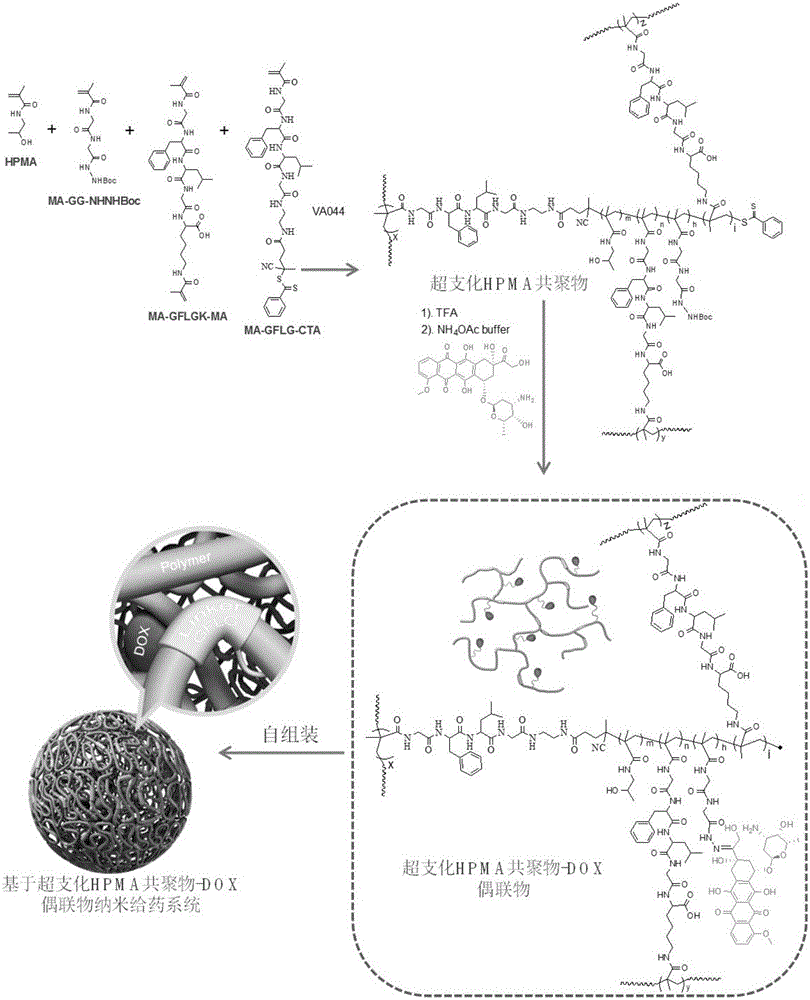
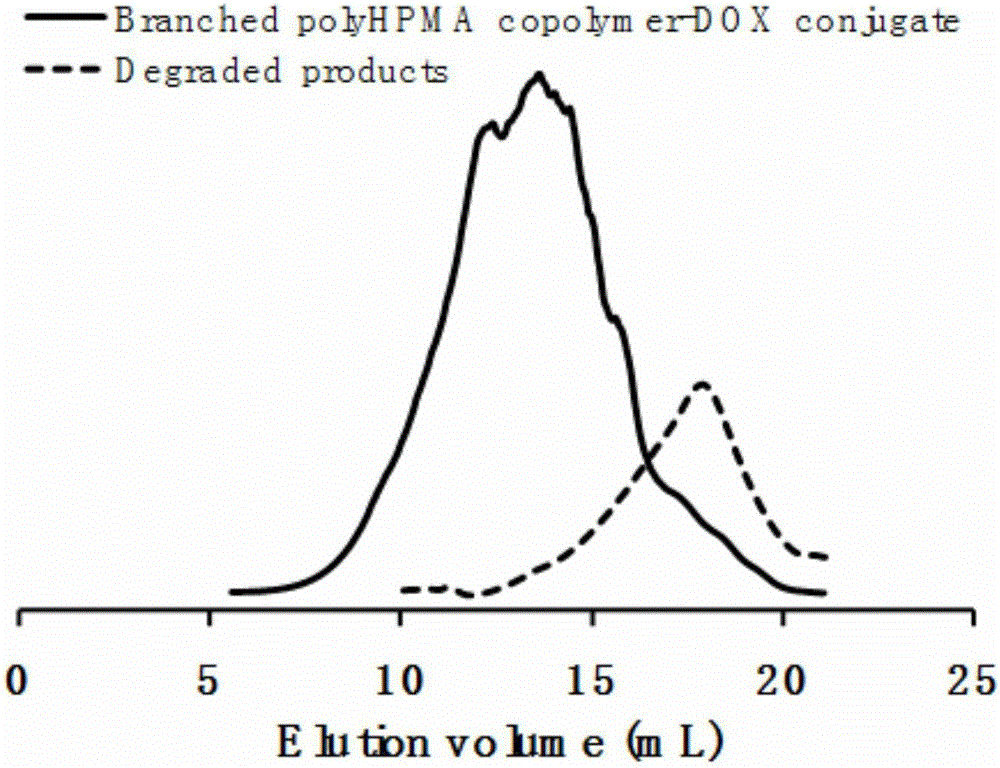
Branched polyHPMA copolymer-DOX conjugate, and preparation method and application thereof
A technology of hyperbranched polymers and copolymers, which can be used in pharmaceutical combinations, pharmaceutical formulations, medical preparations of non-active ingredients, etc. The effect of stable performance, good biocompatibility and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of functionalized chain transfer agent MA-GFLG-CTA
[0045]
[0046] Dissolve MA-GFLG-OH (1.84g, 4mmol), EDCI (764mg, 4mmol), HOBt (676mg, 5mmol) in 50mL of anhydrous DMF, then add DIPEA (3.5mL, 20mmol), under the condition of nitrogen ice bath Stir for 30min. Then N-Boc-ethylenediamine (800mg, 5mmol) was added, stirred in a nitrogen ice bath for 30min, and then stirred at room temperature for 12h. After stirring, another 250 mL of ethyl acetate (EtOAc) was added to the solution, and the water in the solution was extracted with EtOAc (50×3). The resulting new solutions were washed with saturated NaHCO 3 solution, 1M HCl solution, NaHCO 3 Washing with a saturated solution, followed by washing with a saturated HCl solution, followed by drying with anhydrous MgSO4, the solvent was removed with a rotary evaporator, and the remaining product was recrystallized in reagent EtOAc / ether (1:1) at 4°C to give a yield of 80%. (1.93 g, 3.2 mmol) of the MA...
Embodiment 2
[0048] Embodiment 2: the preparation of hyperbranched HPMA copolymer-DOX conjugate
[0049] Such as figure 2 As shown, HPMA (1.36g, 9.5mmol), MA-GG-NHNHBoc (126mg, 0.4mmol), MA-GFLGK-MA (65.6mg, 0.1mmol), MA-GFLG-CTA (29.6mg, 38.8μmol) Dissolve in 7 mL of VA044 (3.6 mg, 12.9 μmol) in a mixed solvent of deionized water / methanol (1:1, v / v).
[0050] The HPMA copolymer is precipitated in acetone / ether (2:1), this operation is repeated twice, and then the HPMA copolymer is separated by centrifugation, and the HPMA copolymer is purified twice by the method of first dissolving and then precipitating, and finally vacuum-dried to obtain Slightly pink powder. Samples further adopted (GE Healthcare) high performance liquid chromatography system combined with Superose6HR10 / 30 chromatographic column (molecular weight range of hydrophilic neutral polymer 15kDa-300kDa / 14mL separation volume), with sodium acetate buffer (pH 6.5) containing 30% acetonitrile as the The mobile phase was fu...
Embodiment 3
[0052] Embodiment 3: the characterization of nanoparticle size, morphology, Zeta potential
[0053] The resulting hyperbranched HPMA copolymer-DOX conjugate was dissolved in double-distilled water to a final concentration of 1 mg / mL, and sonicated for 30 seconds. The size of the aqueous phase and the zeta potential were measured by Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). Subsequently, the solution containing nanoparticles was dropped on a silicon wafer of an appropriate size until the solvent evaporated completely, and the size was further confirmed by field emission scanning electron micro-scope (FE-SEM).
[0054] The results show that: the dynamic light scattering (DLS) method detects that the particle size of the aqueous phase of 1mg / mL hyperbranched HPMA copolymer-DOX conjugate nanoparticle is 102nm, and the particle size dispersion is 0.397, such as Figure 4 Shown in B. The nanoscale size and narrow particle size dispersion indicate that the prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com