Laboratory-scale synthesizing and curing method of terminal carboxyl liquid fluorine elastomer
A technology of fluoroelastomer and synthesis method, applied in the field of small-scale synthesis and curing of carboxyl-terminated liquid fluoroelastomer, can solve the problem of high curing temperature and achieve the effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (a) First add 5L of acetone into a 10L reactor, and add 2000g of vinylidene fluoride-tetrafluoroethylene-hexafluoropropylene copolymer (Zhonghao Chenguang FPM2461) into the reactor one by one under mechanical stirring. After the addition, set the temperature at 30° C., rotate at 300 r / min, and stir for 5 hours to completely dissolve the solid fluoroelastomer.
[0025] (b) Set the temperature to 0°C. After the system in the reactor is cooled to 0°C, add 100 g of benzyltriethylammonium chloride and stir for 10 minutes.
[0026] (c) at first preparation 850g mass fraction is the KOH aqueous solution of 45%, takes by weighing 382.5g solid potassium hydroxide, divides and adds in the large beaker that 467.5g deionized water is housed in cold water, constantly stirs, until all Dissolved without exotherm. Then weigh 800g of 30% hydrogen peroxide solution in another large beaker. Use a peristaltic pump to control the rate of addition of alkali and oxidant. First, add a mass f...
Embodiment 2-5
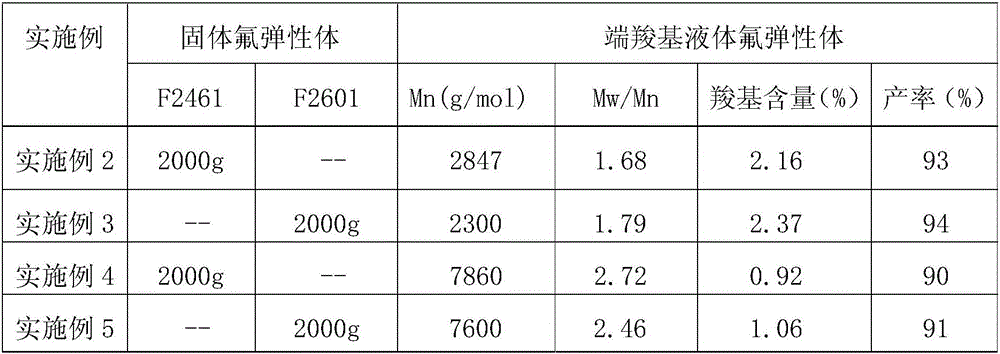
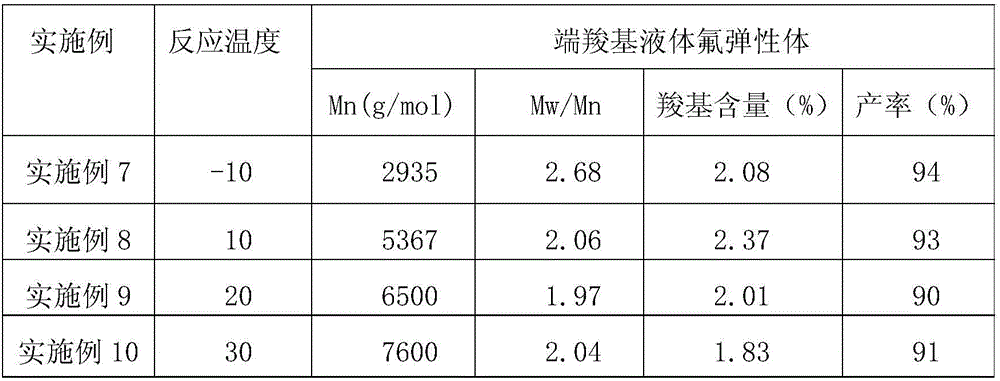
[0030] The specific implementation method is the same as in Example 1, except that the types of raw materials and the reaction temperature are changed. The reaction temperature in Example 2-3 is -10°C, and the reaction temperature in Example 4-5 is 40°C. The specific changes are shown in Table 1, and the results are also listed in Table 1. Carboxyl content was determined by acid-base titration.
[0031]
Embodiment 6
[0033] (a) First add 2.5L of acetone into a 10L reactor, and add 1000g of vinylidene fluoride-perfluoromethyl vinyl ether copolymer (Russian CKΦ260) into the reactor one by one under mechanical stirring. After the addition, set the temperature at 30°C and the rotation speed at 300r / min. After stirring for 4 hours, add 2.5L petroleum ether to make the system in the reactor free of air bubbles. Stir for another hour to completely dissolve the solid fluoroelastomer. Other follow-up steps are with embodiment 1, and charging amount is also with embodiment 1.
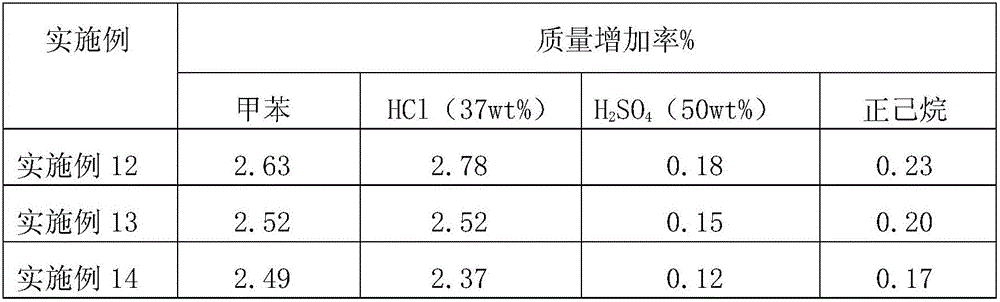
[0034] The resulting product was a pale yellow viscous substance at room temperature. The number average molecular weight is 3081, the molecular weight distribution width is 2.34, and the carboxyl content is 2.163%. 1776cm in the infrared spectrum -1 The characteristic absorption peak at -C=O indicates that the carboxyl-terminated liquid fluoroelastomer was successfully synthesized. The dynamic viscosity at 27°C is 75000cp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
| Onset decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com