Blue photosensitive resin composition, color filter and liquid crystal display device having the same
A resin composition and blue light-sensitive technology, which is applied in the field of blue photosensitive resin composition and liquid crystal display devices, can solve the problems of low exposure and high sensitivity, lower quality of liquid crystal display devices, and failure to solve the problems of color filter adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
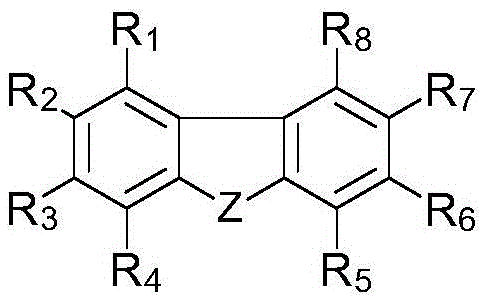
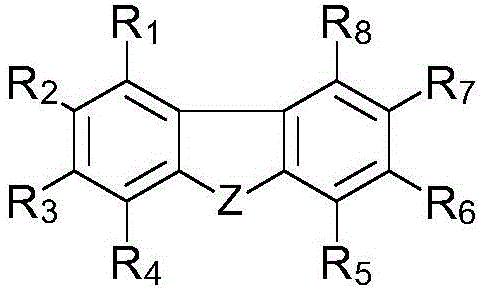
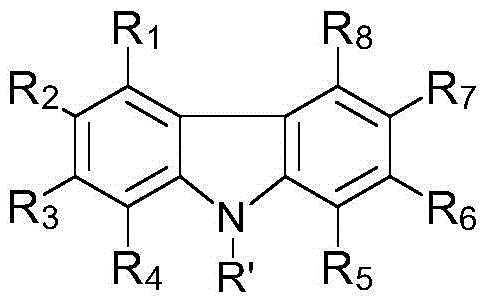
[0342] Synthesis example 1. Synthesis of photopolymerization initiator (C-1): (13-(2-ethylhexyl)-5-(2,4,6-trimethylbenzyl Acyl)-13H-dibenzo[a,i]carbazol-8-yl]-[4-(2,2,3,3-tetrafluoropropoxy)-phenyl]-ketone oxime-O- Acetate synthesis
[0343] As intermediate 13-(2-ethylhexyl)-13H-dibenzo[a,i]carbazole, 1-naphthylhydrazine hydrochloride was used instead of phenylhydrazine, and according to the following steps 1 to 4 disclosed The sequence of synthesis of photopolymerization initiator C-1.
[0344] Step 1: 13-(2-Ethylhexyl)-13H-dibenzo[a,i]carbazole
[0345] 13H-Dibenzo[a,i]carbazole can be produced according to a specific sequence, such as the sequence disclosed in the literature [SYNLETT, 2006, 7, 1021].
[0346] To 13H-dibenzo[a,i]carbazole (0.70 g, 3.22 mol) in DMF (3 ml) was added sodium hydride (0.19 g, 4.67 mmol) at 0°C. After stirring at 0°C for 1 hour, 1-naphthylhydrazine hydrochloride (1.24 g, 6.44 mmol) was added at 0°C, and the mixture was stirred at room te...
Synthetic example 2
[0353] Synthesis example 2. Synthesis of photopolymerization initiator (C-2): 1-(9,9-H-7-nitrofluoren-2-yl)-acetoxime-O-acetate Synthesis
[0354] Step 1: Synthesis of 1-(9,9-H-7-nitrofluoren-2-yl)-ethanone
[0355]
[0356] Dissolve 5.0g (23.7mmol) of 2-nitrofluorene (1) in 100ml of anhydrous nitrobenzene, add 6.31g (47.4mmol) of anhydrous aluminum chloride, raise the temperature of the reactant to 45°C, and slowly A solution obtained by dissolving 2.79 g (35.5 mmol) of acetyl chloride in 30 ml of anhydrous nitrobenzene was added, and the reactant was heated up to 65° C. and stirred for 1 hour. Thereafter, the reactant was cooled to room temperature, 70 ml of distilled water was added and stirred for about 30 minutes, and then the product was filtered. The resulting solid product was dispersed in 50 ml of ether, stirred at room temperature for 30 minutes, filtered and dried to obtain pale yellow 1-(9,9-H-7-nitrofluoren-2-yl)-ethanone (2 ) 5.08 g (84.7%).
[0357] ...
Synthetic example 3
[0363] Synthesis example 3. Synthesis of photopolymerization initiator (C-3): 1-(9,9-diethyl-9H-fluoren-2-yl)-1,2-propanedione-2- Synthesis of Oxime-O-Acetate
[0364] Step 1: Synthesis of 9,9-diethyl-9H-fluorene (2)
[0365]
[0366] Dissolve 200.0 g (1.20 mol) of fluorene (1), 268.8 g (4.80 mol) of potassium hydroxide and 19.9 g (0.12 mol) of potassium iodide in 1 L of anhydrous dimethyl sulfoxide under a nitrogen atmosphere, and keep the reactant at 15°C , and then 283.3 g (2.60 mol) of bromoethane was slowly added over 2 hours, and the reactant was stirred at 15° C. for 1 hour. Afterwards, 2 L of distilled water was added to the reactant, and after stirring for 30 minutes, the product was extracted with 2 L of dichloromethane, and the extracted organic layer was washed twice with 2 L of distilled water, then the recovered organic layer was dried with anhydrous magnesium sulfate, and the solvent was reduced Pressure distillation and fractional distillation of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com