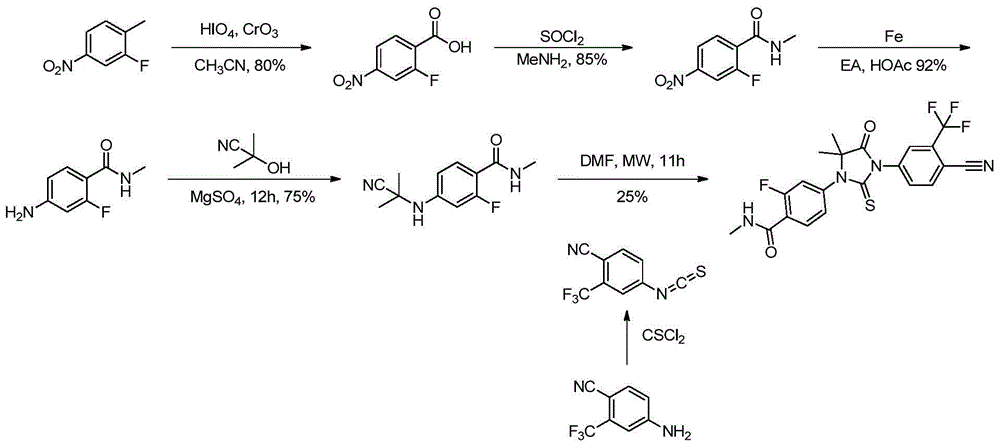
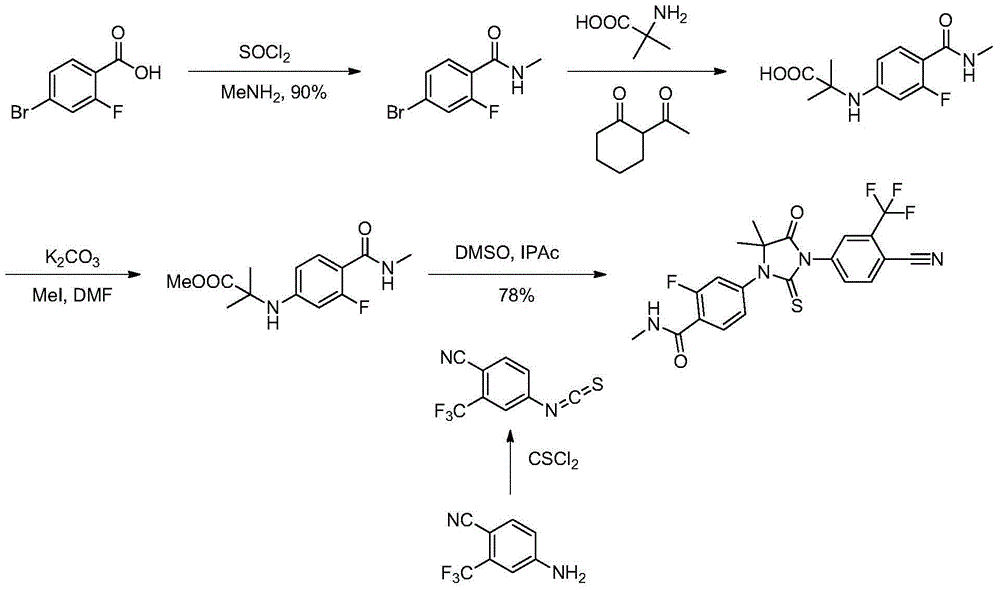
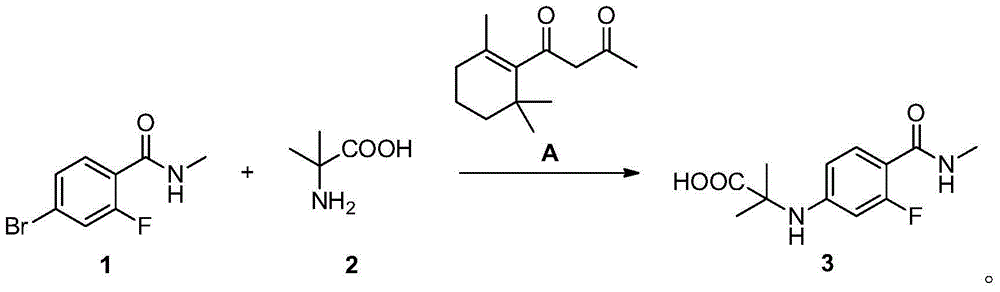
Method for preparing benzamide compound and its intermediate
A technology of benzamide and compound, applied in the field of preparation of benzamide compound, can solve the problems of low safety, complicated operation, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] The preparation of embodiment 1 benzamide compound 3
[0108] In a 3L three-necked flask, add compound 1 (200g, 0.86mol), compound 2 (133g, 1.29mol), cuprous chloride (16.8g, 0.17mol), compound A (20.8g, 0.1mol) and potassium carbonate solid (303.6 g, 2.2 mol), then add 1 liter of DMF and 20 ml of distilled water, and replace nitrogen three times. Turn on the heating and raise the temperature to 115 degrees, TLC detection, after 10 hours, the raw materials completely disappeared. Stop responding. Let cool to room temperature.
[0109] Filter to remove solid insolubles, wash the filter residue with a little DMF, combine the filtrates, evaporate to dryness under reduced pressure, add 400ml of water to completely dissolve the system, and extract twice with 400ml of isopropyl acetate to remove organic impurities in the raffinate. The water phase was stirred, and a saturated citric acid solution was added dropwise therein to adjust the pH of the reaction system to 4-5, an...
Embodiment 2
[0110] The preparation of embodiment 2 benzamide compound 3
[0111] In a 3L three-necked flask, add compound 1 (200g, 0.86mol), compound 2 (133g, 1.29mol), cuprous chloride (16.8g, 0.17mol), compound A (20.8g, 0.1mol) and potassium carbonate Solid (303.6 g, 2.2 mol), then 1 liter of DMF was added, and nitrogen was replaced three times. Turn on the heating and raise the temperature to 115 degrees, TLC detection, after 15 hours, the raw materials completely disappeared. Stop responding. Let cool to room temperature.
[0112] Filter to remove solid insolubles, wash the filter residue with a little DMF, combine the filtrates, evaporate to dryness under reduced pressure, add 400ml of water to completely dissolve the system, and extract twice with 400ml of isopropyl acetate to remove organic impurities in the raffinate. The water phase was stirred, and a saturated citric acid solution was added dropwise therein to adjust the pH of the reaction system to 4-5, and then cooled to b...
Embodiment 3
[0113] The preparation of embodiment 3 benzamide compound 4
[0114] Add benzamide compound 3 (15g, 59mmol) and 75 grams of methanol in a 250ml three-necked flask, add thionyl chloride (45g, 378mmol) dropwise thereto under stirring, add, reflux, and at this temperature Stirring was continued for 6 hours, and the raw material disappeared completely as detected by TLC.
[0115] Concentrate under reduced pressure, distill off the solvent, and then slowly pour the reaction solution into saturated aqueous sodium bicarbonate solution to adjust the pH of the system to 7-8. At this time, a large amount of white solid is precipitated. After filtering, the filter cake was beaten with 80 ml of water for 30 minutes, and filtered again to obtain a white solid, which was vacuum-dried at 60 degrees to constant weight to obtain 15 g, with a yield of 90% and a purity of 98.5% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com