Preparation method of quercetin-3-O-beta-D-glucuronic acid
A technology of glucuronic acid and quercetin, applied in the field of preparation of quercetin-3-O-β-D-glucuronic acid reference substance, can solve the problems of large amount of organic solvent, high cost, high toxicity, etc. Achieve the effect of simple operation, high cost and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
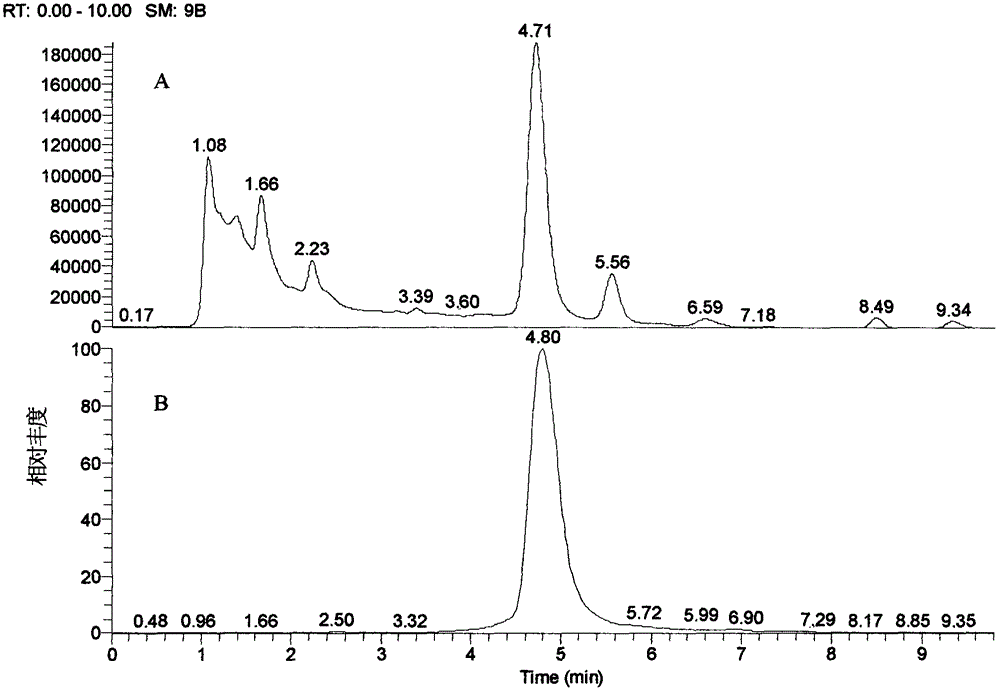
[0020] Take 400g (about 4cm / segment) of the medicinal herbs of the barbangui, add 8 times the amount of water, boil and reflux for 2 times, each time for 1 hour, filter with gauze, concentrate to the equivalent concentration of 0.8g / ml of medicinal materials, add ethanol to adjust the The concentration is 50%, stand at low temperature until the precipitation is complete, the supernatant is filtered, concentrated, the sample is dissolved in 20% methanol, placed on an ODS open chromatography column (diameter 5cm × length 32cm), methanol-0.05% acetic acid water (20: 80→35:65→45:55) gradient elution, each half column volume is 1 elution fraction, each ratio elutes 2 column volumes, 45% methanol eluted fractions are concentrated under reduced pressure, and methanol is reconstituted. Sol, TLC, CHCl 3 -MeOH-H 2 O-HCOOH (7:3:0.5:0.2) was developed, there were obvious dark spots at 254nm (the ratio shift value Rf was about 0.4), the fraction was allowed to stand at low temperature, an...
Embodiment 2
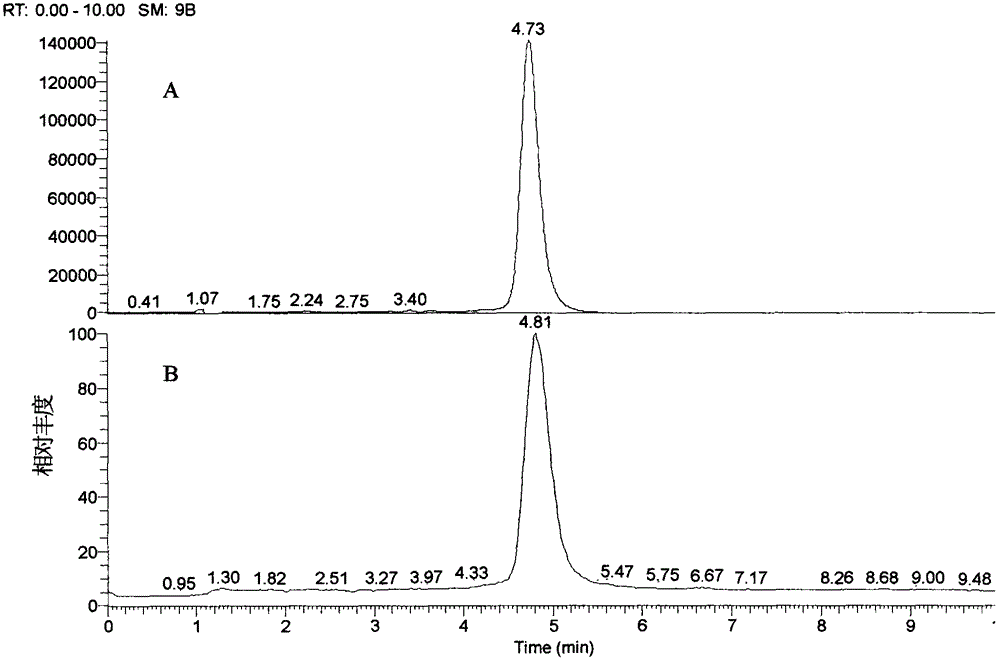
[0024] Take 400g (about 4cm / segment) of the medicinal herbs of the barbangui, add 8 times the amount of 30% ethanol, heat and reflux for 2 times, 1 hour each time, filter with gauze, concentrate to the equivalent concentration of medicinal materials 0.8g / ml, add ethanol Adjust its concentration to 50%, stand at low temperature, wait until the precipitation is complete, filter the supernatant, concentrate, dissolve the sample in 20% methanol, and apply it to an ODS open chromatographic column (diameter 5cm×length 32cm), methanol-water (20:80→ 30:70→40:60) gradient elution, one elution fraction per half column volume, each fraction was concentrated under reduced pressure, reconstituted in methanol, thin chromatography, CHCl 3 -MeOH-H 2 O-HCOOH (7: 3: 0.5: 0.2) was developed, and there were obvious dark spots at 254 nm, which were the target fluid content (ratio shift value Rf was about 0.4), stand at low temperature, a large amount of light yellow powder was precipitated, and th...
Embodiment 3
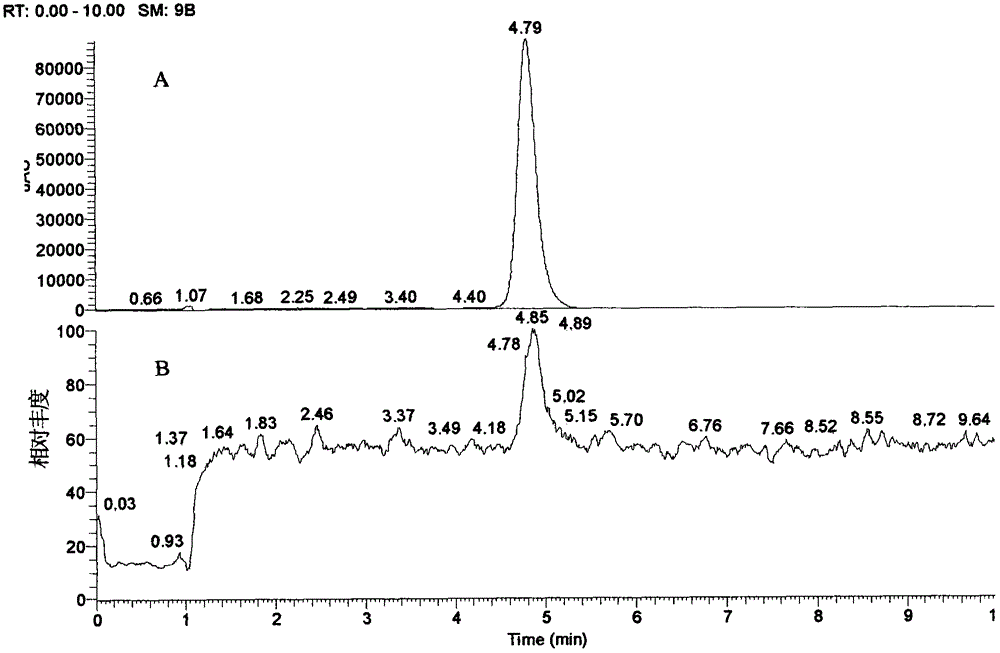
[0026]Take 400g (about 4cm / segment) of the medicinal herbs of the barbangui, add 8 times the amount of water, boil and reflux for 2 times, each time for 1 hour, filter with gauze, concentrate to the equivalent concentration of 0.8g / ml of medicinal materials, add ethanol to adjust the The concentration is 50%, stand at low temperature until the precipitation is complete, the supernatant is filtered, concentrated, the sample is dissolved in 20% methanol, placed on an ODS medium and low pressure chromatography column (diameter 4cm × length 33cm), methanol-0.05% acetic acid water (20 :80→30:70→40:60) gradient elution, 40% methanol elution fractions were concentrated under reduced pressure, reconstituted in methanol, thin chromatography, CHCl 3 -MeOH-H 2 O-HCOOH (7:3:0.5:0.2) was developed, there were obvious dark spots at 254nm (the ratio shift value Rf was about 0.4) and the fraction was left to stand at low temperature. After a large amount of light yellow powder was precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com