Tetracyclic triterpenoid compound and its use in medicines
A compound and drug technology, applied in the field of new medicine research, can solve the problem of no compound anticancer activity found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
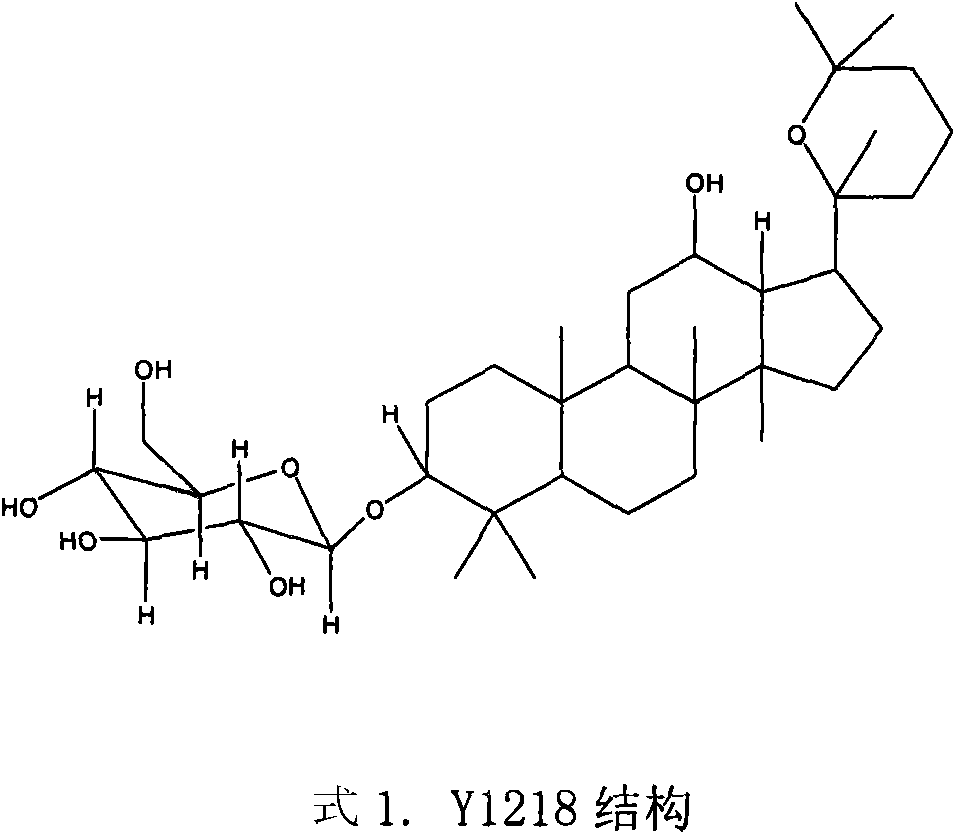
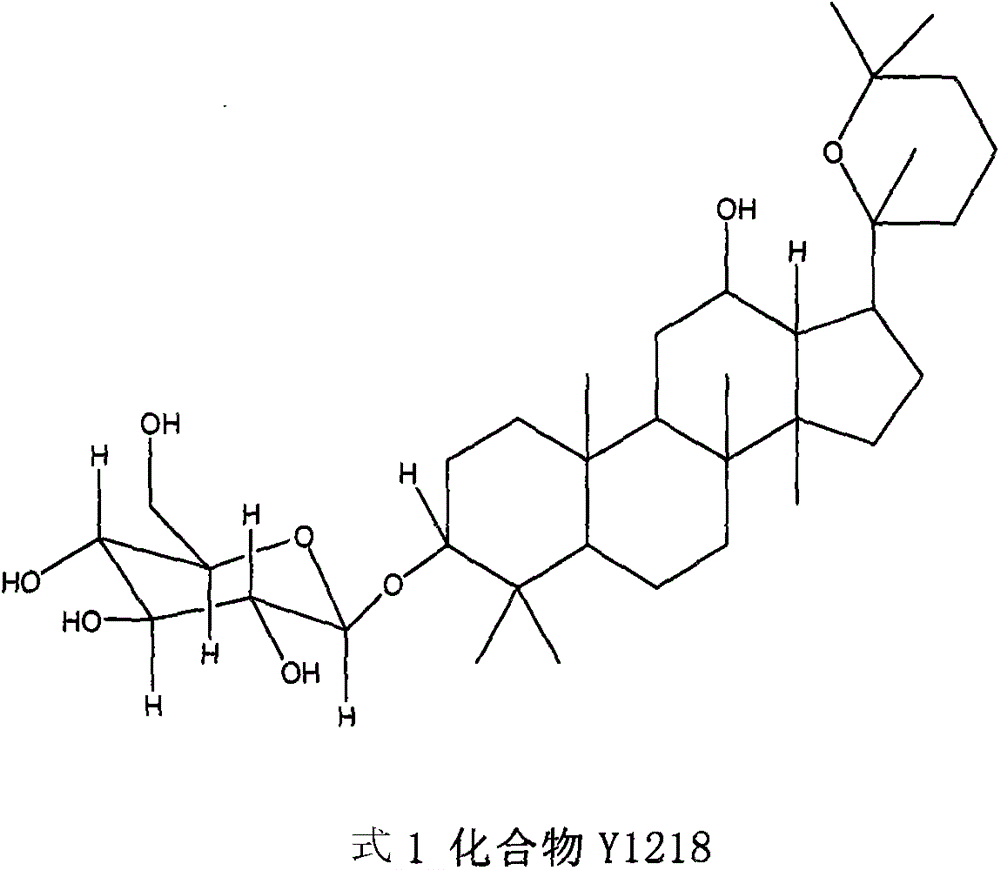
[0016] Preparation of Y1218.
[0017] Routinely pulverize 60 kg of the dried Panax notoginseng crude drug, extract with ethanol, and extract 5 times with 1 liter of ethanol each time, and combine. Evaporate under reduced pressure to constant weight. 2.17 kg of ethanol extract was obtained. The ethanol extract was dissolved in hot water, and sequentially partitioned and extracted with n-hexane, ethyl acetate and n-butanol. Obtained n-butanol extract 0.73kg. The n-butanol extract was repeatedly separated by silica gel H (10-40 μm) column chromatography, and eluted with n-butanol: acetic acid: water = (2-8): (1-4): (3-7) gradient , get Fr1. Fr1 was repeatedly subjected to silica gel column chromatography and eluted with chloroform:methanol=(2-20):1 to obtain 0.78 g (0.013 parts per ten thousand) of Y1218.
Embodiment 2
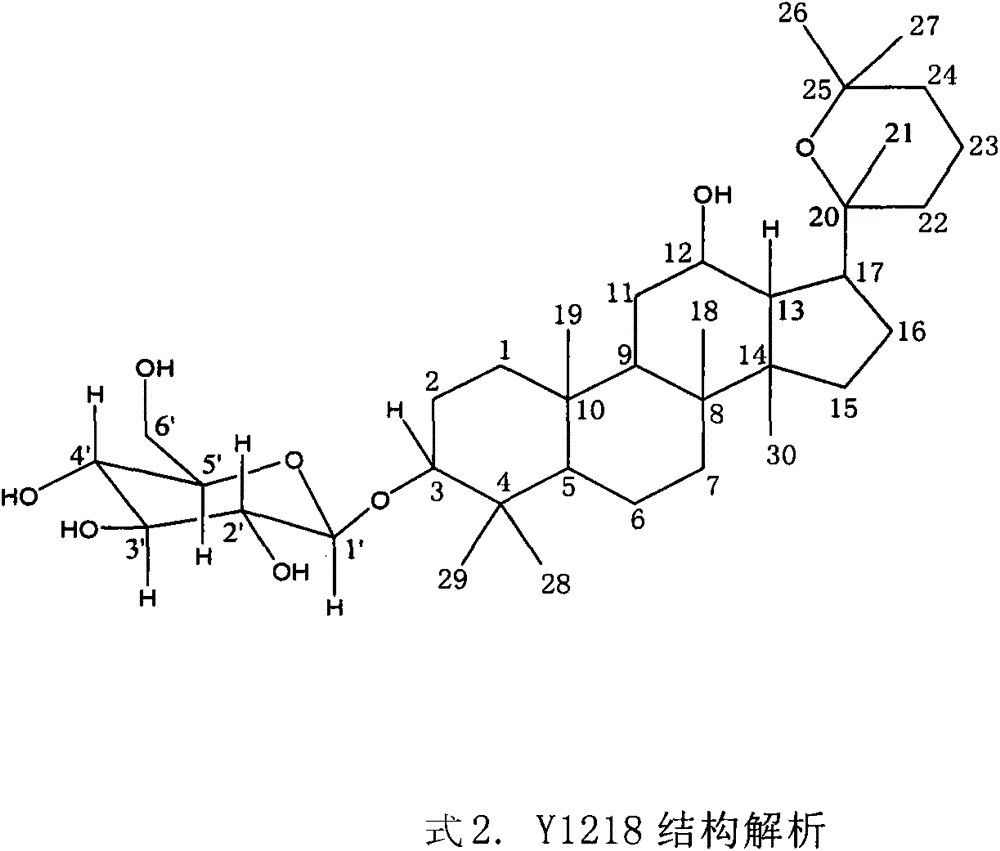
[0019] Structural identification of Y1218.
[0020] Y1218 is white yellow powder. mp166-167; IR (cm -1 )3400, 2971, 1450, 1386; [α] 20 D +3.83°(MeOH; C=0.5); FAB-MS(m / z), 623[M+H] + , the main fragment peak 495[623-128(C 8 h 16 O, sidechain)] + , 461 [623-162 (Glc)] + , suggesting that there is a 6-carbon sugar, so it is speculated that its molecular weight is 622, and its molecular formula is C 36 h 62 o 8 ;Y1218 13 C NMR spectrum data compared with ginsenoside Rh2 except C 20 -C 27 Except for the difference, other data are basically the same. It shows that the basic skeleton of Y1218 is the same as that of Rh2, that is, the basic structure of dammarane type. 1 H MNR appears δ4.96 (1H, d, J=7.84Hz), indicating that the Y1218 molecule contains a β-configured pyranose, and Y1218 is hydrolyzed with hydrochloric acid to obtain D-glucopyranose, confirming that the sugar group is glucopyranose sugar. It is further confirmed that Y1218 and Rh2 except C 20 ~C 27 Exc...
Embodiment 3
[0025] Example 3 In vitro activity of Y1218 on tumor cells
[0026] Inhibitory effect on tumor cells in vitro Experimental tumor cells: metastatic colon cancer SW620, gastric adenocarcinoma BGC-823, etc. The in vitro cytotoxicity was measured by the MTT method, and 200ul of well-grown cells were taken and added to a 96-well plate, CO 2Incubate for 24 hours in the incubator. Add 20 μl / well of the test drug and incubate for 48 hours. Add 20 μl MTT into a 96-well plate, react for 4 hours, absorb the supernatant, add 20 μl DMSO / well, measure the absorbance value of each well at a wavelength of 570 nm with an enzyme-linked immunosorbent detector, and calculate the killing effect on cells. Determination of the concentration of Y1218 inhibiting 50% tumor cell growth (IC 50 ), and compared the strength of the effect with paclitaxel. The comparison results are shown in Table 1.
[0027] Table 1.Y1218 in vitro activity (IC 50 : μl / ml)
[0028] compound SW620 BGC-823 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com