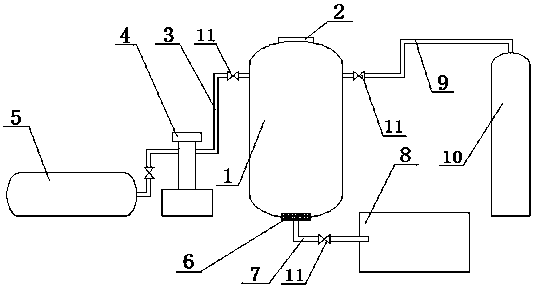
A method for recovering high-purity potassium chloride from fluorination reaction by-products
A technology of fluorination reaction and potassium chloride, which is applied in the chemical field, can solve the problems of small calcium fluoride precipitate particle size filtration, inability to recycle potassium fluoride, high cost and environmental protection issues, etc., to improve solid form and improve utilization The effect of low value and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 1000g of KCl-KF mixed salt (the mass fraction of potassium fluoride is 20%), which has been dried at 500°C for 2 hours, into the autoclave, and dissolve 1200g of liquid ammonia at 20°C and a pressure of 1.3Mpa. Then filter, the filtrate is the liquid ammonia solution of potassium fluoride, and gasification obtains 196g of pure potassium fluoride powder, purity 99%, yield 98%, filter cake is potassium chloride crude product, and filter cake is used 11g water and 69g The mixed solution of ethanol is rinsed, then add about 1560g of water to dissolve the solid, then slowly heat up to about 80 ° C, until the solid is just completely dissolved, then cool to room temperature, and add 3120g of isopropanol for recrystallization to obtain potassium chloride crystals , and then washed with an appropriate amount of isopropanol and dried to obtain 750 g of pure white potassium chloride with a purity of 99.92% and a yield of 93.8%.
Embodiment 2
[0037] Add 1000g of KCl-KF mixed salt (the mass fraction of potassium fluoride is 10%), which has been dried at 500°C for 2 hours, into the autoclave, and add 600g of liquid ammonia to dissolve it at 20°C and a pressure of 1.3Mpa. Then filter, the filtrate is the liquid ammonia solution of potassium fluoride, and gasification obtains 97g of pure potassium fluoride powder, purity 99%, yield 97%, filter cake is potassium chloride crude product, and filter cake is mixed with 13g water and 77g The mixed solution of ethanol is rinsed, then add about 1760g of water to dissolve, then slowly heat up to about 80 ° C, until the solid is just completely dissolved, then cool to room temperature, and add 3520g of isopropanol for recrystallization to obtain potassium chloride crystals, Then wash with an appropriate amount of isopropanol and dry to obtain 850 g of pure white potassium chloride with a purity of 99.95% and a yield of 94.4%.
Embodiment 3
[0039] Add 1000g of KCl-KF mixed salt (the mass fraction of potassium fluoride is 30%), which has been dried at 500°C for 2 hours, into the autoclave, and dissolve 1800g of liquid ammonia at 20°C under a pressure of 1.3Mpa. Then filter, the filtrate is the liquid ammonia solution of potassium fluoride, and gasification obtains 294g of pure potassium fluoride powder, the purity is 99%, and the yield is 98%. The filter cake is the potassium chloride crude product. The mixed solution of ethanol is rinsed, then add about 1370g of water to dissolve, then slowly heat up to about 80 ° C, until the solid is just completely dissolved, then cool down to room temperature, and add 2740g of isopropanol for recrystallization to obtain potassium chloride crystals, Then wash with an appropriate amount of isopropanol and dry to obtain 656 g of pure white potassium chloride with a purity of 99.92% and a yield of 93.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com