Synthetic method for 1,2-di-O-isopropylidene-3,5,6-tri-O-benzyl-D-glucofuranose
A technology of glucofuranose and isopropylidene, applied in 1, can solve the problems affecting the preparation of tribenzyl glucoside, lack of purity, low purity content, etc., and achieve the effect of less impurities, easy operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0036] Example 1: Preparation of 1,2-di-O-isopropylidene-3,5,6-tri-O-benzyl-D-glucofuran
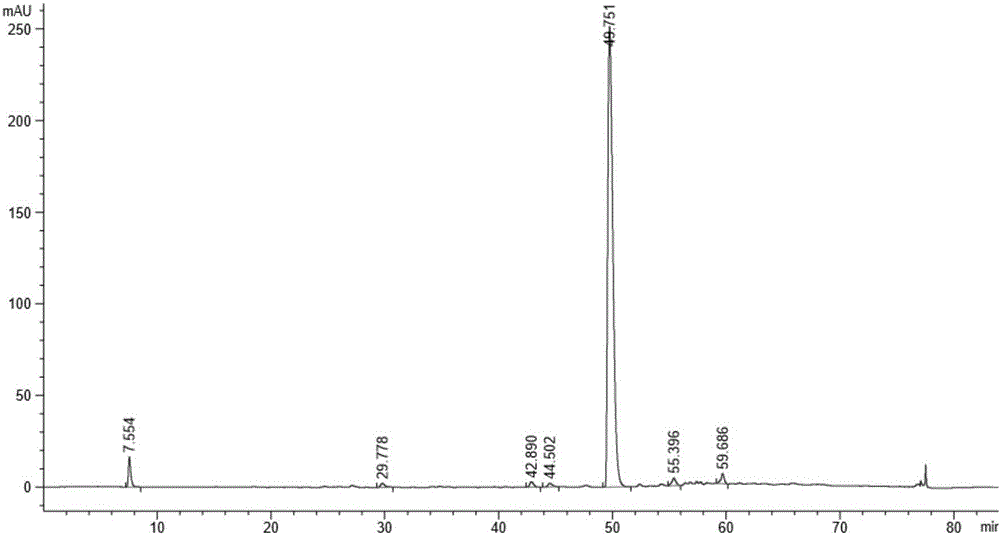
[0037] Add 1,2-di-O-isopropylidene-D-glucofuranose (25g, 0.1135mol) into a 500ml three-necked flask containing benzyl chloride (150g, 1.185mol), heat it in an oil bath, and control the temperature Slowly add solid potassium hydroxide (76.3g, 1.362mol) under stirring at 85-95°C, the reaction is exothermic, control the adding speed so that the internal temperature does not exceed 95°C, and react for 3-4 hours. After the reaction is completed, cool the system down to 20-30°C, add water (100ml) dropwise, and then add n-hexane (100ml), stir for 15 minutes, let stand to separate the layers, and wash the organic layer with water (100ml), then wash at 40°C-45 Distill n-hexane under reduced pressure at ℃ until no flow out, sample HPLC detection, the results are as follows figure 2 shown. .
[0038] Purify the light yellow material after reduced evaporation by molecular distillation under spec...
Embodiment example 2
[0039] Example 2: Preparation of 1,2-di-O-isopropylidene-3,5,6-tri-O-benzyl-D-glucofuran
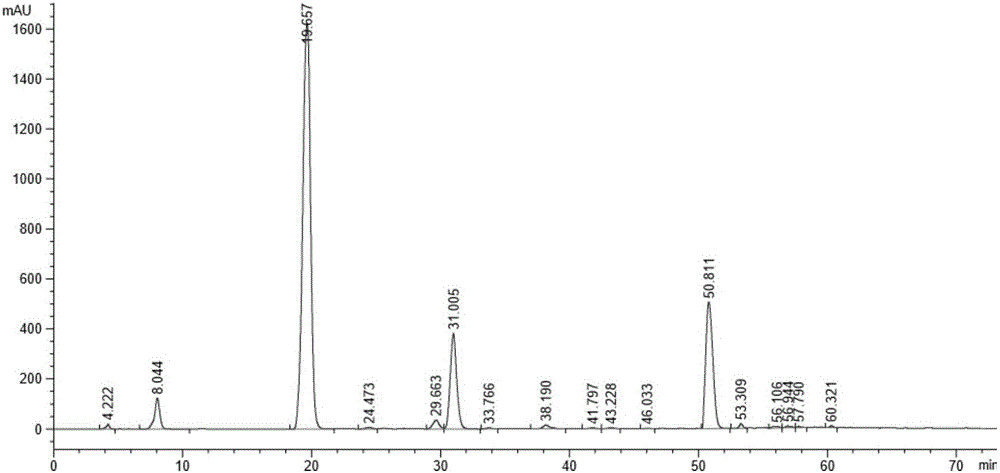
[0040] Add 1,2-di-O-isopropylidene-D-glucofuranose (25g, 0.1135mol) into a 500ml three-necked flask containing benzyl chloride (150g, 1.185mol), heat it in an oil bath, and control the temperature At 85-95°C, slowly add solid sodium hydroxide (56.8g, 1.419mol) under stirring, the reaction is exothermic, control the addition rate so that the internal temperature does not exceed 95°C, and react for 3-4 hours. After the reaction is completed, cool the system down to 20-30°C, add water (100ml) dropwise, and then add n-hexane (100ml), stir for 15 minutes, let stand to separate the layers, and wash the organic layer with water (100ml), then wash at 40°C-45 Distill n-hexane under reduced pressure at ℃ until no flow out, sample HPLC detection, the results are as follows Figure 4 shown.
[0041] Purify the light yellow material after reduced evaporation by molecular distillation under specific...
Embodiment example 3
[0042] Example 3: Preparation of 1,2-di-O-isopropylidene-3,5,6-tri-O-benzyl-D-glucofuran
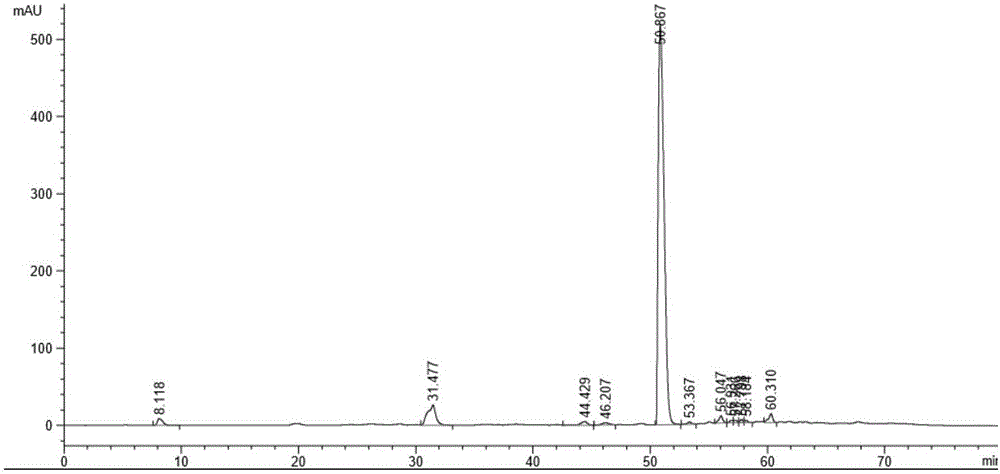
[0043]Add 1,2-di-O-isopropylidene-D-glucofuranose (25g, 0.1135mol) into a 500ml three-necked flask containing benzyl chloride (180g, 1.422mol), heat it in an oil bath, and control the temperature Slowly add solid potassium hydroxide (79.5g, 1.419mol) under stirring at 90-100°C, the reaction is exothermic, control the addition rate so that the internal temperature does not exceed 100°C, and react for 3-4 hours. After the reaction is completed, cool the system down to 20-30°C, add water (100ml) dropwise, and then add n-hexane (100ml), stir for 15 minutes, let stand to separate the layers, and wash the organic layer with water (100ml), then wash at 40°C-45 Distill n-hexane under reduced pressure at ℃ until no flow out, sample HPLC detection, the results are as follows Figure 6 shown. .
[0044] Purify the light yellow material after reduced evaporation by molecular distillation under sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com