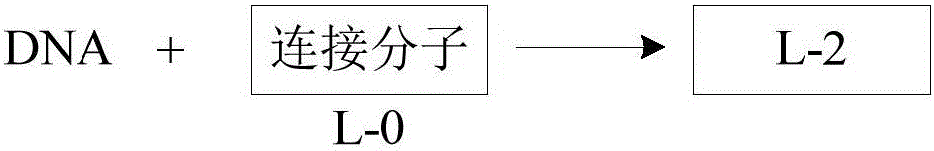Method for solid-phase synthesis of DNA-coded compound database
A solid-phase synthesis, compound library technology, applied in the direction of organic compound library, DNA preparation, recombinant DNA technology, etc., can solve the problems of long cycle, poor solubility, limited synthesis reaction types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] (1), preparation of L-G-1
[0142]
[0143]To G-1 (0.5g, CPG initial load: 32μmol / g, source: Shanghai Lingjiang Industrial Development Co., Ltd.), add 3mL dichloromethane containing compound L-1 (69mg, 0.1mmol; manufacturer: Aldrich) Methane solution was reacted overnight at room temperature; after the reaction was completed, the solvent was removed by filtration to obtain a solid; the solid was washed with DMF (2 mL×3) and dichloromethane (2 mL×3) respectively to obtain L-G-1.
[0144] Take L-G-1, remove the Fmoc protecting group with piperidine, and use the UV absorption quantification of the elimination product of Fmoc to determine that the load of L-G-1 is 14.5 μmol / g, and the yield is 91%.
[0145] (2), preparation of L-2
[0146]
[0147] Dissolve single-stranded DNA (32.0 nmol, molecular weight 7663.9) (the sequence of single-stranded DNA is GGAGCTTGTGAATTCTGGCACTCG) in 30 μL NaHCO 3 To the buffer solution, add 20 ml of L-0 (1.0 mg, 3.0 μmol; manufacturer...
Embodiment 2
[0173] (1) According to the preparation method of L-G-1 in Example 1, L-G-1 was obtained;
[0174] (2) According to the preparation method of L-2 in Example 1, L-2 was obtained;
[0175] (3) According to the preparation method of L-G-2 in Example 1, L-G-2 was obtained;
[0176] (4) According to the preparation method of L-G-2-1 in Example 1, L-G-2-1 was obtained;
[0177] (5) Preparation of L-G-3-1.b
[0178]
[0179] Add 2-ethylphenylisocyanate (1.47 mg; manufacturer: Alfa), triethylamine (5 μL) and DMF (15 μL) to L-G-2-1 (5.0 mg), and react at 25°C to 30°C for 16 hours, The solvent was removed by filtration to obtain a filter cake; the filter cake was washed three times with distilled water and 0.1M TEAA buffer solution respectively to obtain L-G-2-1-1.b;
[0180] Take part of L-G-2-1-1.b (2.0 mg) and add 150 μL of concentrated ammonia water, heat to 55 ° C for 1 hour to remove the solid phase carrier, filter and distill off the solvent under reduced pressure, and wash...
Embodiment 3
[0187] (1) According to the preparation method of L-G-1 in Example 1, L-G-1 was obtained;
[0188] (2) According to the preparation method of L-2 in Example 1, L-2 was obtained;
[0189] (3) According to the preparation method of L-G-2 in Example 1, L-G-2 was obtained;
[0190] (4) According to the preparation method of L-G-2-1 in Example 1, L-G-2-1 was obtained;
[0191] (5) Preparation of L-G-2-2.c
[0192]
[0193]To L-G-2-1 (20mg), add p-aminobenzoic acid (4.15mg, manufacturer: Alfa), 2-(7-azobenzotriazole)-N,N,N',N'-tetra Methylurea hexafluorophosphate (6.9 mg, manufacturer: Alfa), DIEA (20 μL) and DMF (60 μL), stirred and reacted at 25° C. to 30° C. for 16 hours, and filtered to remove the solvent to obtain a filter cake; the filter cakes were respectively Wash three times with distilled water and 0.1M TEAA buffer solution to obtain L-G-2-2.c;
[0194] Take part of L-G-2-2.c (2.0 mg) and add 150 μL of concentrated ammonia water, heat to 55 ° C for 1 hour to remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com