Method for fluorination modification of electrode material
An electrode material and modification technology, applied in the field of new energy materials, can solve the problems of difficult to effectively control the fluorine content, hidden danger of explosion safety, difficult to effectively control, etc., to improve the surface and interface characteristics of the electrode, facilitate the reaction, and suppress the hydrogen The effect of hydrofluoric acid destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
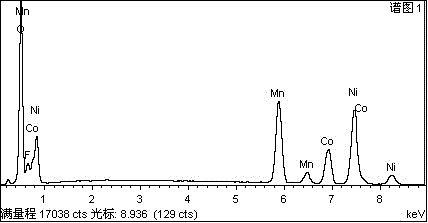
[0021] 4.6 g LiNi 0.6 co 0.2 mn 0.2 o 2 The ternary positive electrode material was added to 0.1 g of 60% polytetrafluoroethylene PTFE aqueous emulsion, diluted with water and stirred at 100 rpm for 2 hours at 50 ° C. After drying, the obtained precursor was placed under the protection of argon at 500 ° C Roasting for 1 hour, LiNi was obtained after cooling 0.6 co 0.2 mn 0.2 o 2 Fluorinated modified product, its EDS spectrum is as follows figure 1 shown. The prepared fluorinated modified electrode material LiNi 0.6 co 0.2 mn 0.2 o 1.95 f 0.05 , Conductive agent SuperP, binder PVDF according to the mass ratio of 8:1:1 to make electrodes, and lithium negative electrodes to make button batteries, charge and discharge tests at 0.1C, the specific capacity of the battery can reach 170mAh / g, 1C The capacity can still be maintained at 156 mAh / g after 50 charge-discharge cycles.
Embodiment 2
[0023] Prepare LiFePO from 10.4 g 4 The positive electrode material was added to 1g of 50% polyvinylidene fluoride PVDF in N-methylpyrrolidone NMP solution, stirred at 500rpm at 70°C for 1h, and the obtained precursor was roasted at 400°C under nitrogen protection for 0.5 hours, cooled LiFePO 4 Fluorinated modified products.
Embodiment 3
[0025] Fully impregnate 10g of graphite powder and 8.7g of liquid fluorine at low temperature, slowly raise the temperature to gradually vaporize the liquid fluorine on the surface of graphite particles, heat at 600°C for 3 hours, adjust the pressure during the reaction, and obtain graphite fluorine after cooling Chemically modified products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com