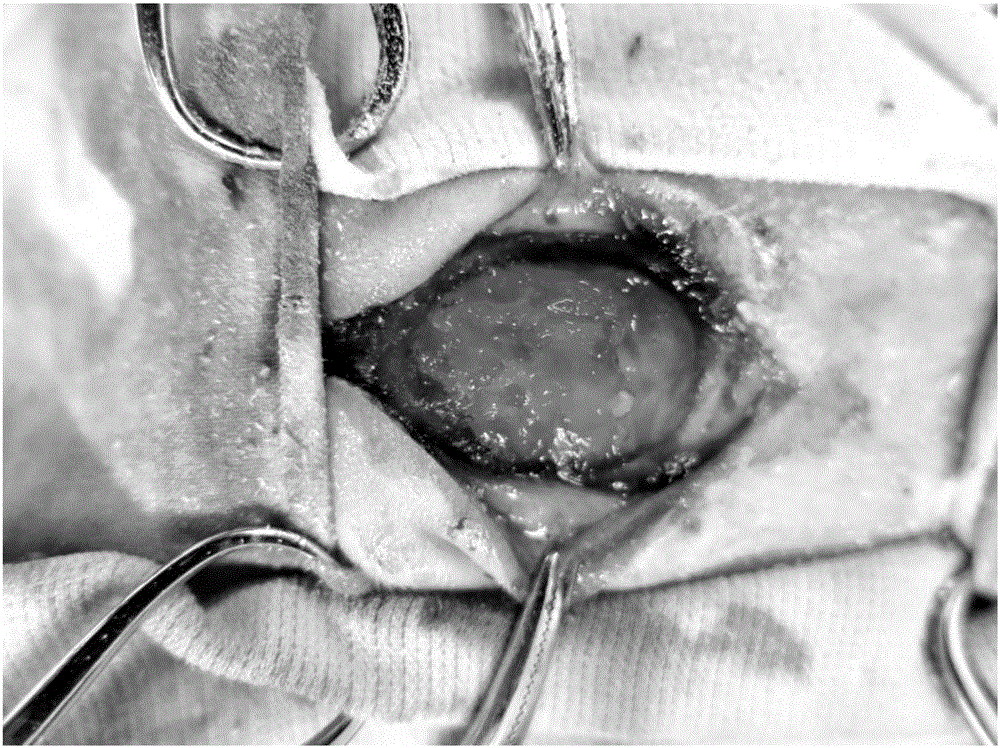
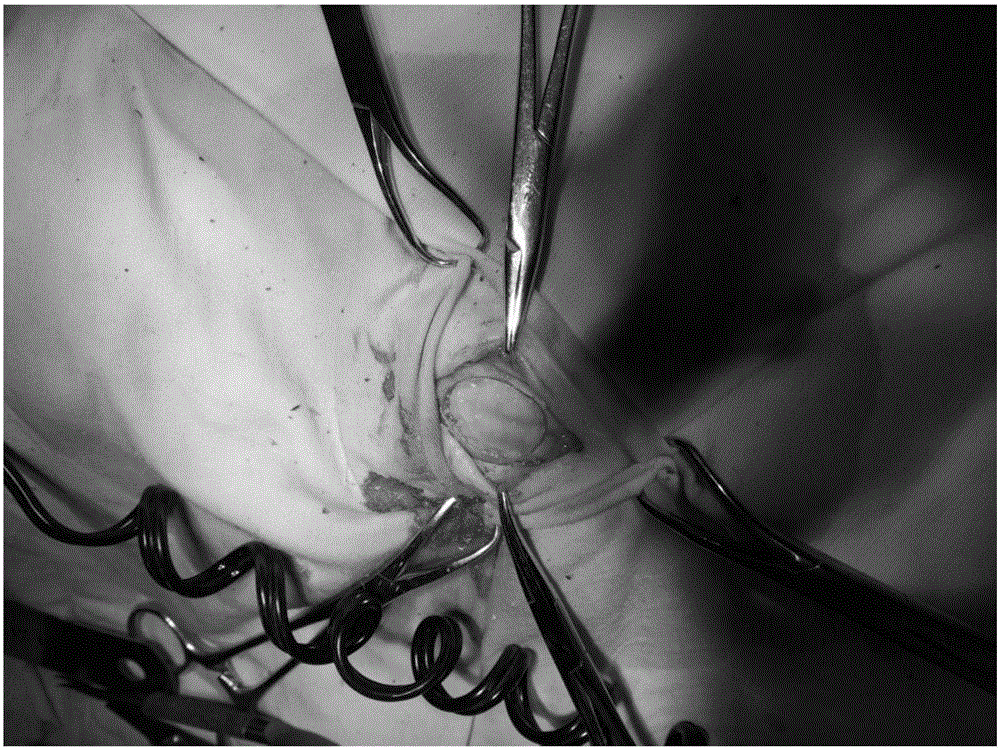
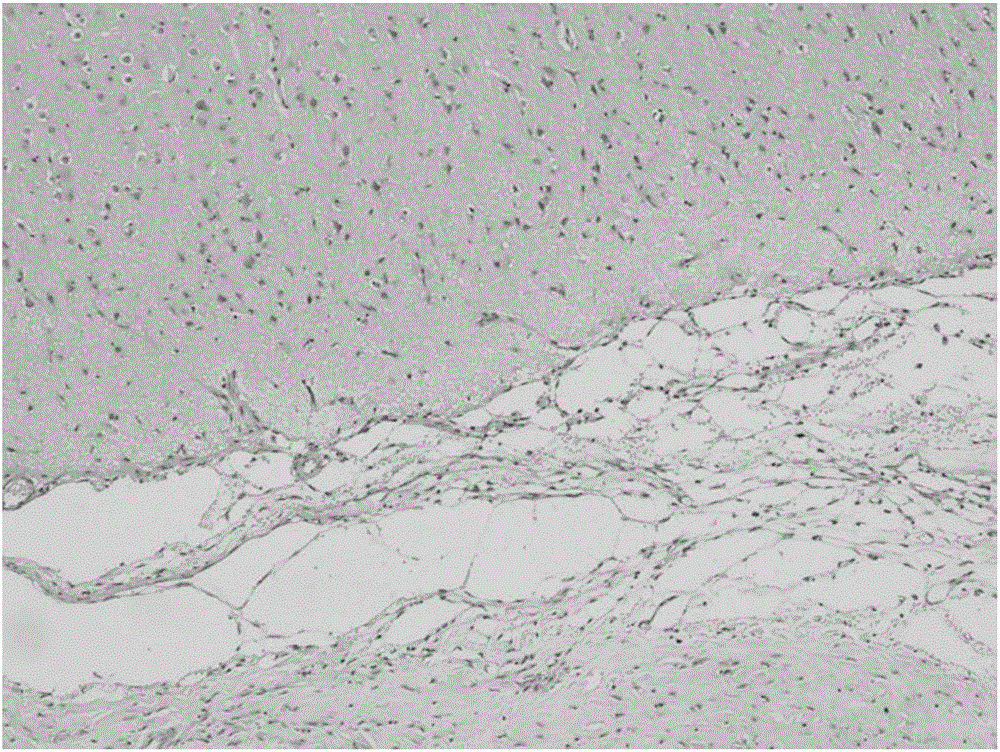
Tissue sealant glue composition, tissue sealant glue, preparation method and application thereof
A technology of sealant and composition, which is applied in the field of biomedicine, can solve the problems of functional effects and use position limitations, and achieve good biocompatibility, high anti-rupture strength, and pain relief effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention provides a tissue sealant composition, a tissue sealant and its preparation method and application. The tissue sealant composition of the present invention mainly includes a first component containing a nucleophile and a second component containing an electrophile , the nucleophilic reagent includes natural substances containing amino groups and / or a first modified product that is modified with a sulfhydryl group on the natural substances containing amino groups, and the natural substances containing amino groups and the first modified products each independently have 10 More than one active group, wherein, the active group is an amino group, a mercapto group; the electrophile includes more than 10 N-succinimides modified on sodium alginate and / or carboxymethyl cellulose The second modified product of the group. Preferably, in the first component, the position where the thiol group is modified is the position of the amino group (eg primary amino gr...
Embodiment 1
[0095]
[0096] 1) Weighing 10mmol (1.6120g) of chitosan with a degree of deacetylation of 87.5%, 15mmol (1.3mL) of mercaptopropionic acid, 20mmol (3.8350g) of soluble carbodiimide, 20mmol (2.3010g) of N-hydroxysuccinimide g), placed in 100 mL of purified water at a temperature of 5° C., and stirred for 8 hours.
[0097] 2) Dialyze the reaction mixture obtained in step 1) in 5 L of normal saline for 12 hours to remove unreacted monomers and small molecular substances, and replace the normal saline every 2 hours.
[0098] 3) Put the mixture obtained by dialysis in step 2) into a freeze dryer for freeze-drying to obtain chitosan modified with sulfhydryl groups, which is the first component.
[0099]
[0100] 1) Weigh 10mmol (2.1610g) of sodium alginate with a viscosity of 500-600mPa·s, 15mmol (2.8760g) of soluble carbodiimide, 15mmol (1.7260g) of N-hydroxysuccinimide, and place in 100mL of Purified water, stirred and reacted for 8 hours.
[0101] 2) Put the reaction mixtur...
Embodiment 2
[0107]
[0108] 1) Weigh 0.4887 poly-L-lysine hydrochloride with a weight-average molecular weight between 3600 and 4300 and a molar percentage of primary amino group content in poly-L-lysine hydrochloride of 35%. g, wherein the molar mass of the primary amino group is 1.5 mmol, and the poly-L-lysine hydrochloride is the first component.
[0109]
[0110] 1) Weigh 15mmol (3.2415g) of sodium alginate with a viscosity of 500-600mPa·s, 15mmol (2.8760g) of soluble carbodiimide, and 15mmol (1.7260g) of N-hydroxysuccinimide. Purified water was stirred and reacted for 10 hours.
[0111] 2) Put the reaction mixture obtained in step 1) into 5 L of normal saline for dialysis for 12 hours to remove unreacted monomers and small molecular substances, and replace the normal saline every 2 hours.
[0112] 3) The mixture obtained by dialysis in step 2) is placed in a lyophilizer for lyophilization to obtain sodium alginate modified with N-succinimide groups, which is the second component...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com