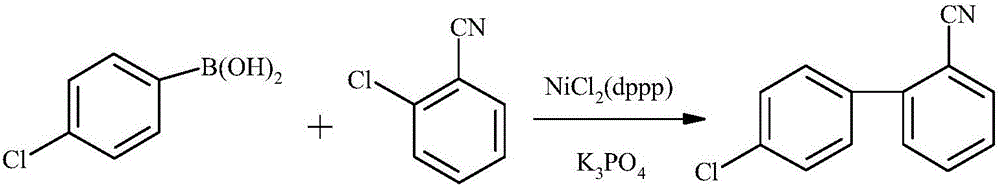
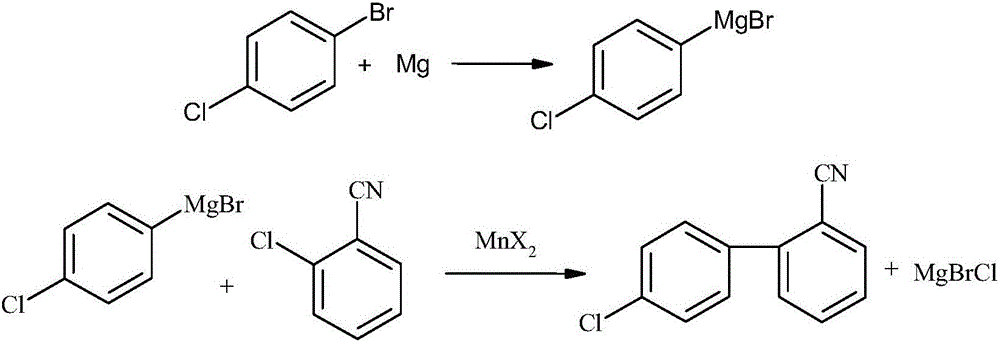
Mass production method for 4-chloro-2'-cyanobiphenyl
A technology of cyanobiphenyl and chlorobromobenzene, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of many pollutants, many by-products, unfavorable large-scale industrial production, etc., and achieve post-processing Simple, less polluting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of Grignard reagent p-tolylmagnesium chloride
[0025] Under nitrogen protection, in a 2000 ml four-neck flask equipped with a reflux condenser, a constant pressure dropping funnel and a stirring device, add 24.3 grams of magnesium powder (1.0 moles), 19.15 grams of 4-chlorobromobenzene (0.10 moles) and 100 Milliliter of tetrahydrofuran, start stirring, heat up to 75°C, add appropriate amount of crystalline iodine to initiate the reaction. A mixed solution of 172.4 g of 4-chlorobromobenzene (0.9 mol) and 900 ml of tetrahydrofuran was added dropwise at the same temperature, and the dropwise addition was completed in 1 hour. Cool to room temperature and set aside.
Embodiment 2
[0026] Embodiment 2: Preparation of 4-chloro-2'-cyanobiphenyl
[0027] Under the protection of nitrogen, in a 2000 ml four-neck flask equipped with a reflux condenser, a constant pressure dropping funnel and a stirring device, add 25.18 g of anhydrous manganese chloride (0.2 mol) and 137.6 g of 2-chlorobenzene Nitrile (1.0 mol) and 200 ml of tetrahydrofuran, stirred, cooled to -5°C in an ice-salt bath, added dropwise the Grignard reaction solution of Example 1, after 1 hour of dripping, the reaction solution was kept at -5-0°C for 2 hours . Recover tetrahydrofuran from the reaction solution under normal pressure, add 500 ml of toluene to the residue, adjust the pH to 2 with 6mol / L hydrochloric acid, separate the layers, wash the organic phase with saturated brine (250 mL*4), dry over anhydrous sodium sulfate, and filter , Toluene was recovered from the filtrate under normal pressure, and the 170-175°C / 0.1MPa fraction was collected by vacuum distillation to obtain 181.6 grams ...
Embodiment 3
[0028] Embodiment 3: Preparation of 4-chloro-2'-cyanobiphenyl
[0029] Under the protection of nitrogen, in a 2000 ml four-necked bottle equipped with a reflux condenser, a constant pressure dropping funnel and a stirring device, add 62.95 g of anhydrous manganese chloride (0.5 mol) and 137.6 g of 2-chlorobenzene Nitrile (1.0 mol) and 200 ml of tetrahydrofuran, stirred, cooled to -5°C in an ice-salt bath, added dropwise the Grignard reaction solution of Example 1, after 1 hour of dripping, the reaction solution was kept at -5-0°C for 2 hours . Recover tetrahydrofuran from the reaction solution under normal pressure, add 500 ml of toluene to the residue, adjust the pH to 2 with 6mol / L hydrochloric acid, separate the layers, wash the organic phase with saturated brine (250 mL*4), dry over anhydrous sodium sulfate, and filter , toluene was recovered from the filtrate under normal pressure, and the 170-175°C / 0.1MPa fraction was collected by vacuum distillation to obtain 183.7 gra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com