Method for clean production of chromium sesquioxide by using chromite as raw material
A technology of chromium trioxide and clean production, applied in the direction of chromium trioxide, chromium oxide/hydrate, etc., which can solve the problems of high energy consumption and high preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
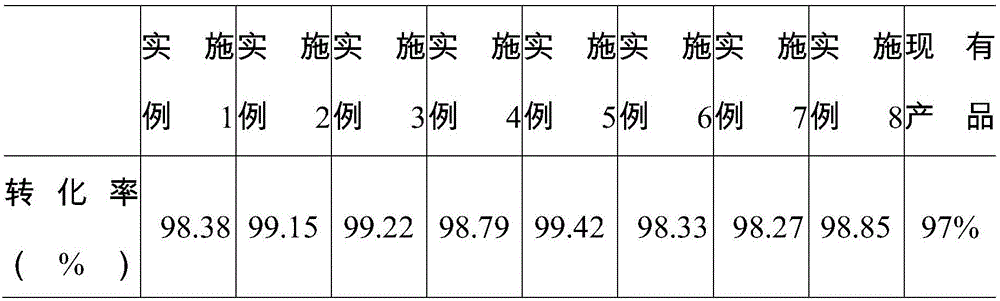
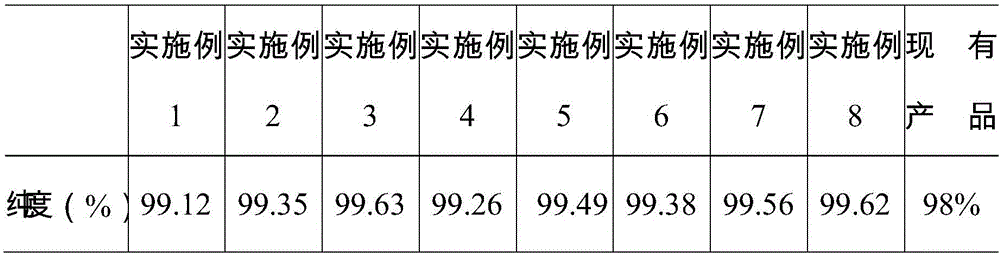
Examples
Embodiment 1
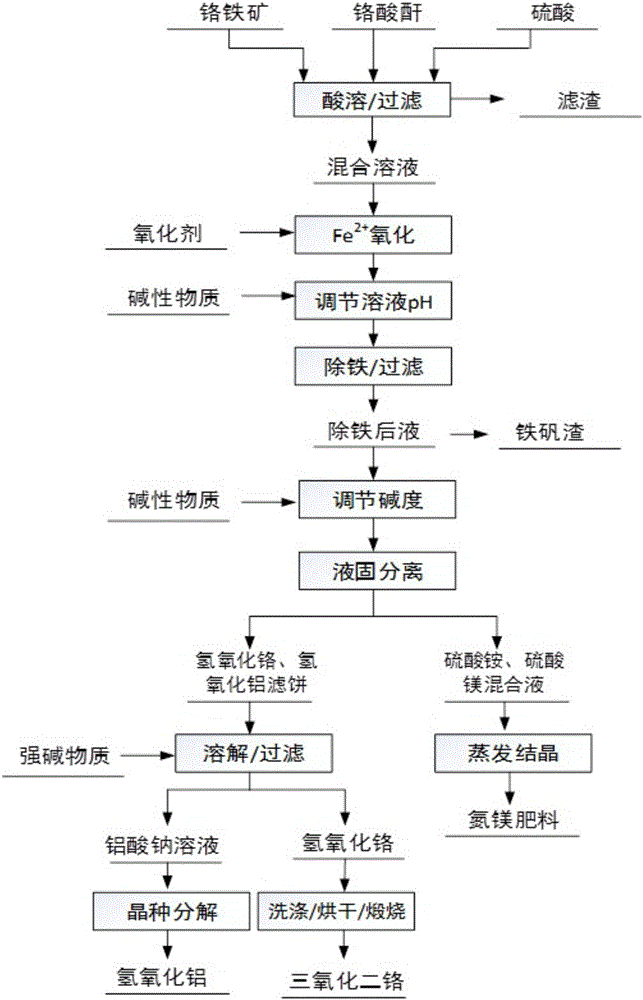
[0027] The production method of present embodiment chromium trioxide is as figure 1 Shown, it is prepared according to the following steps:
[0028] One, take by weighing 200g chromite ore, calculate the amount 380g of the vitriol oil required for reaction according to the content of metal element chromium in the chromite ore, and dilute the vitriol oil required to concentration to be 58%;
[0029] 2. Add the dilute sulfuric acid, chromite and 20g of chromic anhydride prepared in step 1 into the autoclave in turn, react at 125°C for 1 hour, and separate the solid and liquid. The obtained liquid phase is a mixture of chromium sulfate, iron sulfate, magnesium sulfate and aluminum sulfate solution;
[0030] 3. Dilute the Fe in the mixed solution with air and hydrogen peroxide that do not introduce other impurities 2+ All oxidized to Fe 3+ , adjusting the pH value of the mixed solution to 2.7 with ammonia water containing ammonium ions;
[0031] 4. Add the mixed solution treat...
Embodiment 2
[0036] Chromium trioxide of the present embodiment is prepared according to the following steps:
[0037] One, take by weighing 200g chromite ore, calculate the amount 380g of the concentrated sulfuric acid needed for reaction according to the content of metal element chromium in the chromite ore, and dilute the required concentrated sulfuric acid to concentration and be 80%;
[0038] 2. Add the dilute sulfuric acid, chromite and 20g of chromic anhydride prepared in step 1 into the autoclave successively, react at 120°C for 1.2h, and separate the solid and liquid. The obtained liquid phase is a mixture of chromium sulfate, iron sulfate, magnesium sulfate and aluminum sulfate. mixture;
[0039] 3. Dilute the Fe in the mixed solution with oxygen that does not introduce other impurities 2+ All oxidized to Fe 3+ , adjusting the pH value of the mixed solution to 2.8 with ammonium bicarbonate containing ammonium ions;
[0040] 4. Add the mixed solution treated in step 3 into an a...
Embodiment 3
[0045] Chromium trioxide of the present embodiment is prepared according to the following steps:
[0046] One, take by weighing 200g chromite ore, calculate the amount 380g of the vitriol oil needed for reaction according to the content of metal element chromium in the chromite ore, and dilute the vitriol oil required to concentration to be 70%;
[0047] 2. Add the dilute sulfuric acid, chromite and 20g of chromic anhydride prepared in step 1 into the autoclave in turn, react at 130°C for 3 hours, and separate the solid and liquid. The obtained liquid phase is a mixture of chromium sulfate, iron sulfate, magnesium sulfate and aluminum sulfate solution;
[0048] 3. Dilute Fe in the mixed solution with oxygen, air and hydrogen peroxide that do not introduce other impurities 2+ All oxidized to Fe 3 + , adjusting the pH value of the mixed solution to 3 with ammonia water or ammonium bicarbonate containing ammonium ions;
[0049]4. Add the mixed solution treated in step 3 into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com