Method for screening melanin formation resistant medicines on basis of magnetic bead separation
An anti-melanin, magnetic bead separation technology, applied in material separation, analytical materials, measurement devices, etc., can solve the problems of unreported, time-consuming, changing the binding properties of proteins and compounds, and achieve reasonable process design and good reproducibility. , the effect of shortening the screening time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of tyrosinase-bonded magnetic beads
[0029] Take a certain volume of magnetic beads, use an equal volume of 25mM MES (pH 6) solution to wash twice, twice for 10min, add an equal volume of freshly prepared EDC solution and NHS solution (50mg / ml) to the washed magnetic beads, Mix well and incubate with slow shaking for 30 min at room temperature. After incubation, put the tube on the magnet for 4 minutes, remove the supernatant, and finally wash twice with 25mM MES (pH6) solution, and set aside.
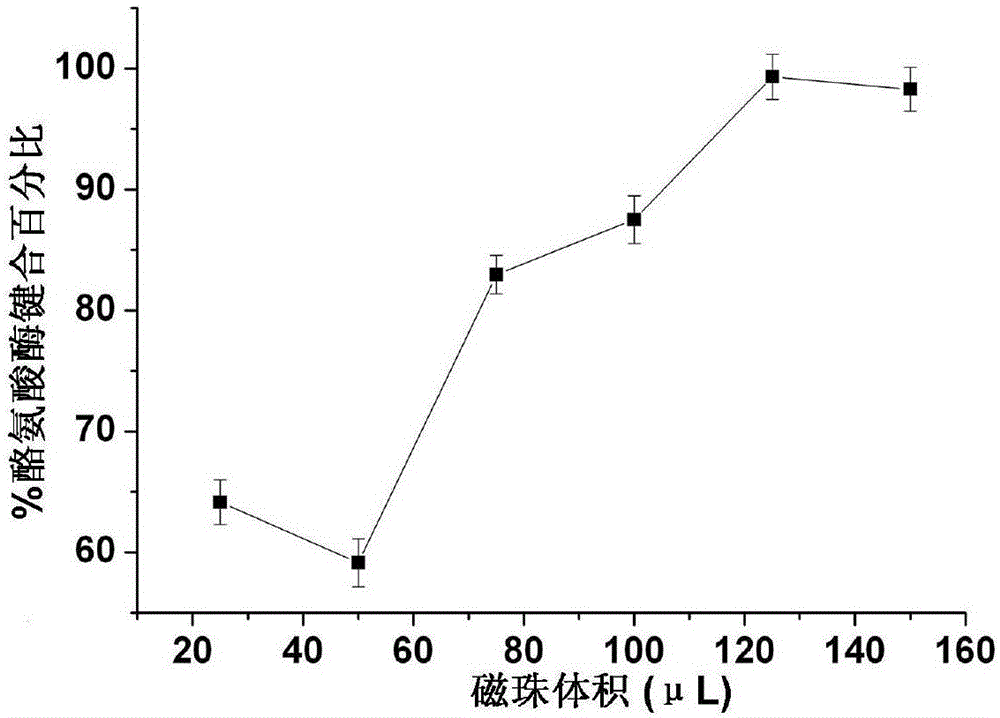
[0030] Take 25, 50, 75, 100, 125, and 150 μL of activated magnetic beads respectively, add 100 μL of tyrosinase (1 mg / ml) solution, shake and mix well, and incubate overnight at room temperature. After incubation, place the tube on the magnetic beads for 4 minutes, remove the supernatant remaining from the binding, and use the Bradford method to determine the protein content in it. Add 100 μL of 0.5% BSA solution to the bonded magnetic beads and shake fo...
Embodiment 2
[0036] Example 2 Research on various factors of the drug screening method for anti-melanin production based on magnetic bead separation
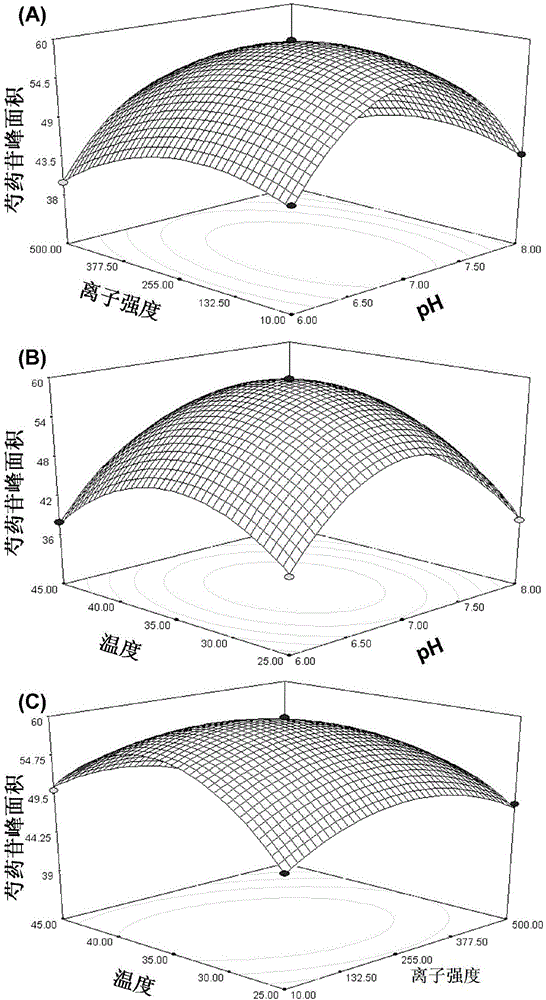
[0037] (1) Using paeoniflorin as a model drug, optimize the denaturation and elution solvent
[0038] Take 20 μL of tyrosinase-bonded magnetic beads prepared in Example 1 above, add 20 μL of paeoniflorin reference solution (0.1 mg / ml) and 160 μL of PBS buffer solution (pH7.4) and mix, and incubate for 30 min. After the incubation is completed, Wash 4 times with 200 μL PBS buffer solution, and the eluate enters the liquid phase for analysis. Finally, the volume concentration is 10%, 30%, 50%, 70% and 90% acetonitrile-water solution (v / v) and the volume concentration is 10%. , 30%, 50%, 70% and 90% methanol-water solution (v / v) for denaturation elution, and the denaturation eluate was taken for liquid phase analysis.
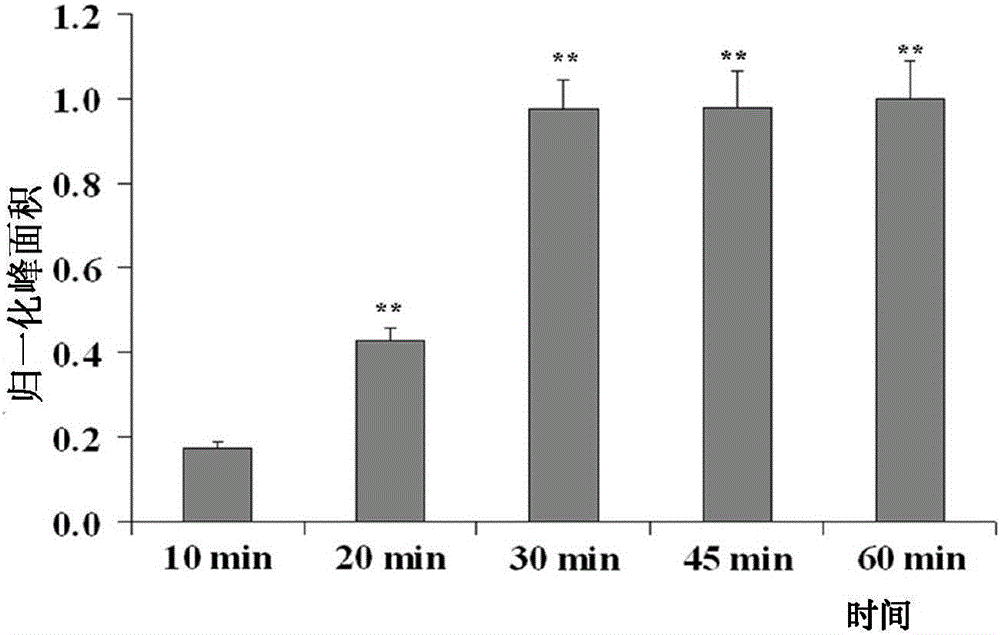
[0039] (2) Using paeoniflorin as a model drug, optimize the incubation time
[0040] Take 20 μL of tyrosinase-bonded magneti...
Embodiment 3
[0053] Example 3 Screening of Anti-Melanin Production Drugs in Sanbai Decoction Components
[0054] (1) Take a certain volume of magnetic beads, wash them twice with an equal volume of 25mM MES buffer solution, each time for 10min, after washing, add an equal volume of 50mg / ml EDC solution and 50mg / ml NHS solution, mix well, and store at room temperature Incubate with slow shaking, after incubation, place on the magnet for 4min, remove the supernatant, and then wash with 25mM MES buffer solution to obtain activated magnetic beads, set aside;
[0055] (2) Take 125 μl of the activated magnetic beads obtained in step (1), add 100 μg of tyrosinase, shake and mix evenly, incubate at room temperature, after incubation, place on the magnetic beads for 4 minutes, and remove the remaining supernatant for bonding , to obtain tyrosinase-bonded magnetic beads;
[0056] (3) Weigh 5g Radix Paeoniae Alba, 5g Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizoma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com