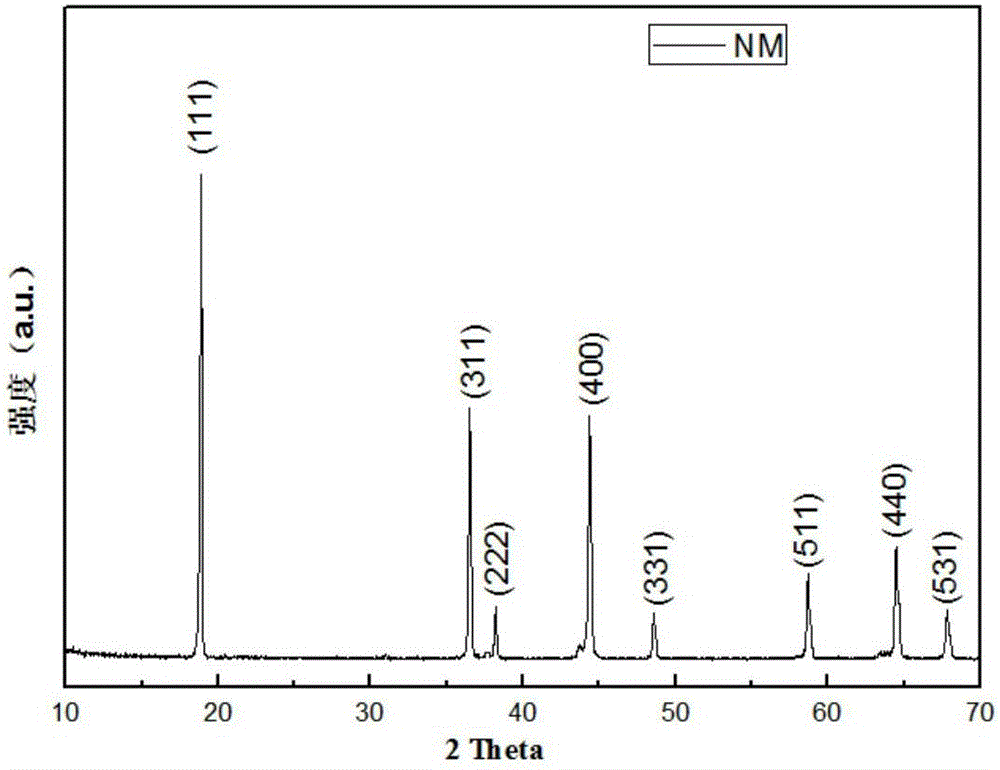
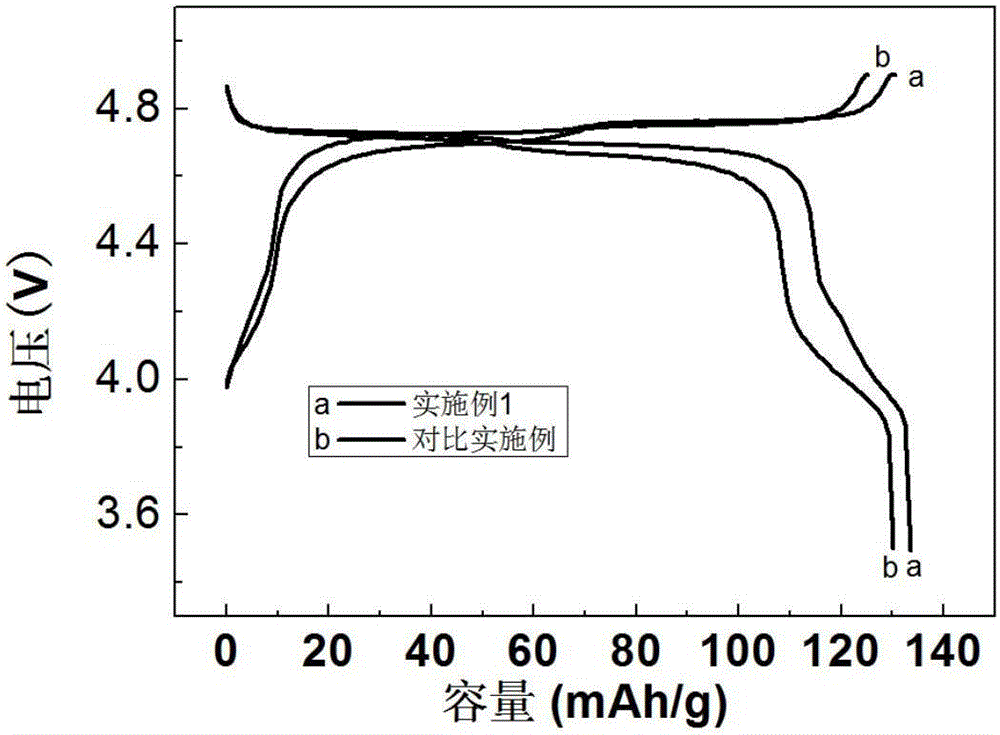
Method for coating lithium nickel manganese oxide with composite
A technology of lithium nickel manganese oxide and composite materials, which is applied in the field of battery material modification and preparation, can solve problems such as difficult to accurately control product composition and reaction rate, poor electrochemical cycle performance, and large drying shrinkage, so as to improve cycle and rate performance , Protect structure and thermal stability, reduce the effect of impurity phase formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for composite material coating lithium nickel manganese oxide, comprising the following steps:
[0028] 1) Preparation of lithium nickel manganese oxide precursor: Accurately weigh lithium acetate, nickel sulfate, and manganese sulfate according to the element Li:Ni:Mn molar ratio of 2:1:3, completely dissolve them in deionized water, and slowly add oxalic acid solution , in which the molar ratio of oxalic acid to nickel sulfate is 4:1, and a precipitate is formed. Under the condition of stirring, the reaction is continued at 50°C for 1h, and the slurry is dried at 80°C. After grinding, it is pre-calcined at 400°C for 3h. , take out after natural cooling and grind to obtain lithium nickel manganese oxide precursor;
[0029] 2) Preparation of lithium nickel manganese oxide precursor suspension: ultrasonically disperse the lithium nickel manganese oxide precursor in isopropanol, and stir to obtain a lithium nickel manganese oxide precursor suspension with a solid...
Embodiment 2
[0032] Controlled trials.
[0033] The preparation of lithium nickel manganese oxide positive electrode material comprises the following steps:
[0034] 1) Preparation of lithium nickel manganese oxide precursor: Accurately weigh lithium acetate, nickel sulfate, and manganese sulfate according to the element Li:Ni:Mn molar ratio of 2:1:3, completely dissolve them in deionized water, and slowly add oxalic acid solution , in which the molar ratio of oxalic acid to nickel sulfate is 4:1, and precipitates are formed, and the reaction is continued at 50°C for 1h under stirring conditions; the slurry is dried at 80°C, and pre-fired at 400°C for 3h after grinding , take out after natural cooling and grind to obtain lithium nickel manganese oxide precursor;
[0035] 2) Lithium nickel manganese oxide cathode material: the pure-phase lithium nickel manganese oxide precursor was calcined at 750°C for 4 hours in an air atmosphere, and then cooled to 500°C for 4 hours for annealing treatm...
Embodiment 3
[0041] A method for composite material coating lithium nickel manganese oxide, comprising the following steps:
[0042] 1) Preparation of lithium nickel manganese oxide precursor: Accurately weigh lithium nitrate, nickel nitrate, and manganese nitrate according to the element Li:Ni:Mn molar ratio of 2.02:1:3, and completely dissolve them in deionized water, slowly add oxalic acid solution , wherein the molar ratio of oxalic acid to nickel nitrate is 5:1, and a precipitate is formed, and the reaction is continued at 60°C for 2h under stirring conditions; the slurry is dried at 85°C, and pre-fired at 450°C for 3.5 hours after grinding. h, take out after natural cooling and grind to obtain lithium nickel manganese oxide precursor;
[0043] 2) ultrasonically disperse the lithium nickel manganese oxide precursor in absolute ethanol, and stir to obtain a lithium nickel manganese oxide precursor suspension with a solid content of 35%;
[0044] 3) The stoichiometric ratio of bismuth ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com